Introduction
The Pathology Screen is a unique institution in Germany that offers high quality translational, pathological analysis of mouse mutants. Located on the campus of the GSF, one of the biggest facilities for small laboratory animal housing in Germany, our technicians have many years of experience with mouse pathology. The supervision by a highly qualified human pathologist together with supportive veterinarians and motivated, in mouse pathology experienced staff, the Pathology Screen of the GMC is a unique combination with no comparison in Germany. During the last four years the Pathology Screen proofed to be a central resource of the German Mouse Clinic. Comparable institutions in Europe, which can offer all modern ancillary techniques, used in human pathology, to be applied to the high throughput analysis of mouse mutants to perform golden standard, comprehensive pathological analysis are rare. Based on the workflow within the GMC, up to 26 mutant mouse lines (1,500 mice) are analyzed in the primary screen per year. Secondary and tertiary screens (for up to 1,500 mice per year) are conducted in collaboration with the mouse provider to assess biological pathways. Our aim is the comprehensive macroscopic and microscopic analysis of mice, including determination of body weight, standardized section and histological examination of the mice to give as much information to the researchers as possible.
Following a complete dissection, an X-ray of the complete bone structure is taken, when indicated. All organs were fixed in 4% buffered formalin and embedded in paraffin for histological examination. Two-#m-thick sections from skin, cervical lymph nodes, thymus, spleen, trachea, lung, heart, salivary glands (parotic gland, submandibular gland, and sublingual gland), esophagus, fore stomach, glandular stomach, duodenum, appendix, colon, liver, pancreatic gland, kidney, urinary bladder, ovary, uterus, vagina/testis epididymis, spermatic cord, brain, cerebellum, adrenal glands, thyroid gland, and parathyroid gland.were cut and stained with hematoxylin and eosin (H&E). Depending on the mouse model additional sections are prepared (i.e. gall bladder, accessory sexual glands, bone, muscle, joints, etc.). Digital pictures are taken to archive the results. Additionally we offer a series of special histochemical stains to examine specific compartments of the body (Giemsa, Massonís trichrome, Luxol Fast Blue, van Gieson, PAS, Prussian Blue, Gram, Ziehl Neelson, chloroacetatesterase etc.). We also perform high quality X-rays, electron microscopy (transmission and laser scanning), and established explicitly for murine, formalin-fixed and paraffin-embedded tissue a broad antibody panel (currently about 110), which steadily increases. For specific selection of tissue areas micro dissection can be done. Molecular analysis as Western Blot, Immunoprecipitation, FISH analysis, PCR analysis in combination with fragment analysis, and LOH analysis can be offered on request.
Due to the high level of quality and many successful cooperations the demand of the Pathology Screen is constantly increasing. We have numerous national and international collaboration partners.
Project Status
The Pathology Screen is established and proofed its worth to the German Mouse Clinic. We are steadily improving our methods and searching for the latest available techniques useful for the detection of morphological phenotypes of mouse mutants. We participate successfully in the international effort to define disease entities in the mouse which includes the descriptions and criteria for diagnosis and establishment of a standardized nomenclature for mouse pathology. In addition, the Pathology Screen is an active member of the EU funded project Eumorphia, where we have the leading role in defining the standards and providing the latest insights for an international consortium. Results of the phenotypic analysis are published together with the mouse provider. Besides own research interests, several manuscripts concerning mutant mice were recently accepted for publication (1-5). In addition the German Mouse Clinic introduced itself to the broad research community in June 2005 (6).
Two Examples for Recently Finished Projects
An interesting ENU (N-ethyl-N-nitrosourea) derived mutant line, named Ali5 ("Ali" for altered limbs), with an autoimmune phenotype was thoroughly analyzed in our screen for morphologic alterations. Our results together with previously performed genome analysis gave hints to the mutated gene, which was finally identified as Plcg2. The Ali5 mutant mouse line provides a mouse model for systemic lupus erythematosus (SLE; see ref. 4).
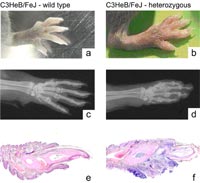
a, b swollen and inflamed paw of affected mouse compared to its litter mate.
c, d Corresponding x-rays. Note severe osteolysis of digital bones and changes in the small joints of the digits.
e, f Histological analysis revealed a severe chronic and acute inflammation.
Another recently published paper addresses the development of acute myeloid leukemia in a murine BM transplantation model. The AML1-ETO fusion gene functionally collaborates with a length mutation of FLT3 (FLT3-LM) in the induction of acute myeloid leukemia (see ref. 5).
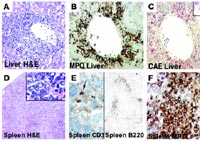
A-C MPO and CAE positive infiltrate in the liver.
D-F affected spleen with few infiltrating reactive T- and B-cells; MPO, myeloperoxidase; CAE, N-acetyl-chloroacetate esterase.
Ongoing Projects
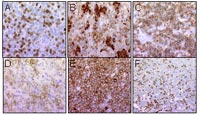
Currently there are twelve secondary screenings ongoing and four manuscripts in preparation. One addresses the results of our endeavour to establish a diagnostic antibody panel for murine hematopoietic tumour tissue.
Outlook
The strong demand of analyzing murine tissues results in a continuous increase of new national and international collaborations. Consequentially, a steep incline of our work emerged and an expansion of our group is highly aspired. We will keep on improving our techniques, increasing out mouse specific antibody panel for formalin-fixed and paraffin-embedded tissue and offering special analysis (e.g. Gene Scan, fluorescence in situ hybridisation, laser capture microdissection) on request. In addition we will continue with training young veterinarians, who are interested in mouse pathology to build a bridge between veterinarians and human pathologist to achieve the maximum outcome of the results.
Lit.: 1. Sorensen KD et al. Mutation of all Runx (AML1/core) sites in the enhancer of T-lymphomagenic SL3-3 murine leukemia virus unmasks a significant potential for myeloid leukemia induction and favors enhancer evolution toward induction of other disease patterns. J Virol. 2004 Dec;78(23):13216-31. 2. Schofield P et al. Pathbase: a new reference resource and database for laboratory mouse pathology. Radiat Prot Dosimetry. 2004;112(4):525-8. 3. Sorensen KD et al. Distinct roles of enhancer nuclear factor 1 (NF1) sites in plasmacytoma and osteopetrosis induction by Akv1-99 murine leukemia virus. Virology. 2005 Apr 10;334(2):234-44. 4. Yu P et al. Autoimmunity and inflammation due to a gain-of-function mutation in phospholipase C gamma 2 that specifically increases external Ca2+ entry. Immunity. 2005 Apr;22(4):451-65. 5. Schessl C et al. The AML1-ETO fusion gene and the FLT3 length mutation collaborate in inducing acute leukemia in mice. J Clin Invest. 2005 Aug 1;115(8):2159-2168 6. Gailus-Durner V et al. Introducing the German Mouse Clinic: open access platform for standardized phenotyping. Nat Methods. 2005 Jun;2(6):403-4.


