Introduction
Dysfunctions of metabolic regulation can cause significant health problems. Their genetic background is largely unknown, and evidence based medical treatment is lacking. One example is the increasing propensity of obesity, which is a risk factor for the development of diabetes and cardiovascular diseases. A recent estimate of the WHO indicates that already 6% of the public health budget is required for obesity related medications in developed countries. Estimates for the heritability of human obesity vary strongly (20-70%) but identification of the polygenes involved in this disease has just started (1). On the other hand, multiple genes have been identified to play a role in the control of energy balance in transgenic mouse models (2).
The Metabolic Screen at the GMC was established in 2002 and provides a comprehensive analysis of metabolic phenotypes in mouse lines and mouse mutants (3). On the basis of standardized procedures, key parameters of energy balance are analysed including food intake, energy assimilation, metabolic rate, body weight, body temperature and locomotor activity. Phenotypic variations of these parameters in mutants and inbred mouse lines are identified with major emphasis on disturbances in body weight regulation, control of energy balance, thermoregulation and body temperature, aiming to identify genes involved in mammalian bioenergetics. Inbred strains used as origin for mutant lines are analysed for basic metabolic informations. Relevant mutant lines may then serve as model organisms for the development, analysis and evaluation of medical treatments for metabolic dysfunctions.
Project Status
Long standing experience in metabolic physiology of our laboratory in Marburg (Philipps University Marburg) was exploited to set-up a comprehensive metabolic screen to search for disorders of energy balance in mutant mouse models at the GMC. This metabolic screen is performed in three steps. A ‘primary screen’ is part of the standard phenotyping program at the GMC. The more detailed ‘secondary’ and ‘tertiary screen’ are based on the results of the primary screen and are prepared for research cooperations with other labs.
Primary Screen: Mice are phenotyped in a standardized procedure, using seven males and seven females, aged 18 ± 1 week. The mice are housed at an ambient temperature of 23 ±1°C and a light:dark cycle of 12:12 h. They are kept singly on grid panels, which allow collection of feces, for a total observation period of two weeks. During the first week mice are fed ad libitum. Body weight, rectal temperature and food intake are measured daily. In parallel, feces production and fecal energy content is monitored in three day intervals to calculate the average daily metabolizable energy. Energy content of food and feces are determined by bomb calorimetry (IKA Calorimeter C7000). During the second week mice are exposed to negative energy balance by restriction to 60% of ad libitum food intake. Measurements of body weight, body temperature and metabolizable energy are continued in order to test whether the normal set of physiological responses is displayed in response to this challenge of energy balance.
Secondary Screen: Individually caged mice are placed inside an open respiratory system and their oxygen consumption and carbon dioxide production are measured at different ambient temperatures. Up to six mice can be measured in parallel. Data are monitored and stored continuously by a computer system. Simultaneous measurement of oxygen consumption and carbon dioxide production allows the determination of the respiratory quotient and thus gives further insight into the preferentially oxidised substrates. It further allows the determination of the thermoneutral zone (ambient temperature range with no need for thermoregulatory heat production), the measurement of basal and resting metabolic rate, energy allocated to activity and thermal conductance. Components of the calorimetry device have been developed and built at Philipps-University of Marburg (PUM) and were transferred to the GMC.
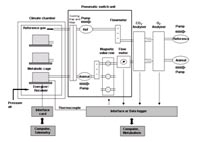
The metabolic system is extended by a telemetry system for recordings of body temperature and locomotor activity patterns. Transmitters implanted into the abdominal cavity allow continuous recordings of body temperature in unrestrained mice. Both systems are running in parallel and provide a complete monitoring of ultradian and circadian patterns of body temperature, heart rate, activity and daily energy expenditure. Like in the primary screen these properties are first determined in mice with ad libitum food supply and then analysed under food restriction. Energetic and thermal responses of mice to this challenge are analysed, and compared with metabolic regulation during ad libitum feeding.
Tertiary Screen: Following the secondary screen the body composition of ad libitum fed and food restricted mice is analysed if required. Body composition of mice is measured using the Soxhlet lipid extraction method with petrol ether after drying the bodies. The water content, the amount of fat and the fat-free dry mass can be deduced and gives further information about the relations between energy uptake, metabolism, fat storage and body composition. In addition body composition can also be measured during treatments by non-invasive DEXA (Piximus). The calibration of the DEXA system has been established at PUM.
Phenotyping at the GMC can be extended by further measurements at PUM, especially for the analysis of endocrines and the expression of genes relevant for the regulation of energy balance.
The mutant lines subjected to the analysis of their metabolic phenotype are provided by other labs of the NGFN consortium, originate from the ENU mutagenesis program at the GSF or from the GGTC. The metabolic laboratory contributes basic measures of energy balance to the primary screen. In addition cooperations are established in which mice are subjected to a more detailed analysis in the secondary and tertiary screen, e.g. mice from Dr. Dirk Isbrandt from Neuronet Hamburg.
This strategy has already been successful. The metabolic screen has so far phenotyped 39 different mutant and six inbred mouse lines in the primary screen, of which 10 lines (mutants or inbreds) were investigated in more detail in the secondary screen. Over all lines 16 new metabolic phenotypes were found. Known metabolic phenotypes were confirmed in two ENU mutant lines showing defects in tooth development. Notably, one mouse mutant exhibiting a clear neurological ataxic phenotype, we discovered an increased basal metabolic rate. This exemplifies that the GMC has the potential not only to discover individual new phenotypic disorders in mouse mutants, but also serves to characterize pleiotropic syndromes caused by single gene defects.

Current projects
Control of energy balance and body weight are some of the large white spots in physiology. We are therefore focussing on mouse lines with special properties in metabolic regulation. For this purpose it was important to characterize several inbred mouse strains which are widely used for generation of mutant lines (Fig. 2). We found fundamental differences in energy uptake and food assimilation indicating the importance of inbred phenotyping when investigating metabolic diseases. A publication describing basic energetic properties of several inbred strains is in progress. In collaboration with the department of comparative medicine of the GSF and several screens of the Mouse Clinic we investigate the influence of housing conditions on mice. Therefore mice housed in Isolated Ventilated Cages (IVC) are compared to the conventional open lid cages indicating a statistical impact on metabolic properties. Data from this project will be published soon. Another project investigates the reproducibility of metabolic phenotyping within the Mouse Clinic, hence the comparability between pure inbred mice with wildtype littermates of mutant strains.
The joint efforts of the GMC and PUM, in collaboration with Ingenium Pharmaceuticals have already been successful in the establishment of the new mouse line SMA1 characterized by semi-dominant dwarfism, GH deficieny, hyperghrelinemia and obesity (4, 5). A heavy weight mutant mouse line generated in the ENU mutagenesis program appear to be of even greater relevance for human obesity. Due to the methodological capacities within the German Mouse Clinic there is a close cooperation with the Neuronet Marburg, working on “Obesity and related disorders”. In a further collaboration with the NGFN project at PUM the genetic background of weight regulation is studied. In one of these cooperations we investigate the alterations in hypothalamic gene expression in relation to diet induced
obesity.
Outlook
The comparative results obtained from primary screening of different inbred strains underlined the necessity for further standardized measurements in the secondary and tertiary screen. Hence, next to interesting mutant phenotypes, inbred mice will be continuously measured to create a further platform of metabolic properties of different mouse lines. Animals of the above mentioned heavy weight mutant mouse line and their littermate controls at the age of 18 weeks are currently measured in the primary and secondary screen, while another batch of that strain will also be exposed to temperature alterations or specific diets.
Because some mouse strains react on food restriction with hypometabolic adaptation followed by hypothermia, this adaptation certainly influences blood gas values like pH, pO2, pCO2 and bicarbonate concentration determined in the Clinical Chemical Screen of the GMC. It is of great interest to add these parameters for blood investigation with focus on metabolic demands. The measurements just started and will intensify the close collaboration with the clinical chemical screening laboratory.
Lit.: 1. Hebebrand J et al. Perspectives : molecular genetic research in human obesity. Obes Rev. 2003 Aug 4(3) : 139-46. Review. 2. Valet P et al. Understanding adipose tissue development from transgenic animal models. J Lipid Res. 2002 Jun;43(6):835-60. Review. 3. Gailus Durner V et al. Introducing the German Mouse Clinic : open access platform for standardized phenotyping. Nature Methods 2 (6): 403-404.4. Meyer CWE et al. A novel missense mutation in the mouse growth hormone gene causes semidominant dwarfism, hyperghrelinemia and obesity. Endocrinology 2004 145(5): 2531-2541. 5. Meyer CWE et al. Gene or Size: Metabolic Rate and Body Temperature in Obese Growth Hormone-Deficient Dwarf Mice. Obesity Research 2004 12(9): 1509-1518.


