Introduction
Sporadic cancer, as it occurs in man, develops from a single cell harboring an initial lesion and progresses in an otherwise normal environment through the accumulation of genetic and epigenetic changes into a highly malignant state. There is a need for accurate mouse models which represent systems for the understanding of human tumorigenesis and the evaluation of innovative tumor therapies. Most experimental tumor studies are performed with transplantation tumors in rodents, which however, do not reflect the clinical situation of cancer formation. In contrast to focal primary tumors, where usually oncogene activation is seen only in a rare number of cells, in conventional transgenic mice an oncogene may be widely expressed in a given tissue. It would be desirable to have a mouse model, where tumor induction can be temporally and spatially controlled. In addition, it is advantageous to be able to titrate the number of transformed cells and to monitor tumor growth using non invasive imaging techniques. Conditional gene expression systems now provide tools to satisfy the demand for mouse models which closely mimic the clinical conditions. The cre/loxP system from bacteriophage P1 is widely used in transgenic animals to induce cre recombinase mediated site specific recombination between loxP sites leading to gene activation or inactivation.
We are using conventional and conditional tumor models, where the expression of the well-known oncogene T antigen (Tag) from Simian Virus 40 (SV40) is directed to the liver or the pancreas leading to the formation of hepatocellular carcinoma (HCC) or insulinoma. Simultaneously, Tag represents a tumor antigen. This gives the opportunity for the development of immunological strategies for the treatment of cancer like the transfer of Tag-specific T cells or vaccination regimens. To be able to monitor tumor growth and therapeutic success we are creating new transgenic reporter mice expressing luciferase (Luc). This allows the visualization of tumor nodules in vivo using luciferase-catalyzed bioluminescence imaging.
Mammacarcinomata
Results
Insulinoma Model
RIP.Tag mice express the oncogene Tag under control of the rat insulin promotor in the beta-islets of the pancreas. Tag expression starts at the age of 10 weeks and leads over the formation of hyperplastic islets to the development of solid insulinoma at about 25 weeks of age (Hanahan et al., 1985). Transfer of activated Tag-specific T cells into tumor-bearing RIP.Tag mice remains ineffective, because the tumor-endothelial barrier is changed in such a way that the lymphocytes fail to infiltrate the tumor. However, after creation of a proinflammatory milieu in the pancreatic islets either by irradiation of the mice or application of immunstimulatory CpG oligodesoxynucleotides prior to transfer the tumor barrier is opened, T cell infiltrate exceedingly, and the tumors are eradicated (Ganss and Hanahan, 1998; Garbi et al., 2004). These data suggest that for a clinically successful immunotherapy the tumor-endothelial barrier needs to be modified. To monitor tumor growth in vivo RIP.Tag mice will be crossed to the newly generated RIP.Luc mice to visualize tumor nodules by bioluminescence imaging. This method allows an easy surveillance of the curative approach and gives the unique opportunity to adapt the treatment regimen to the individual mouse to ensure the highest possible therapeutic success.
Conditional liver tumor models
To develop an inducible mouse model for autochthonous liver tumors, we generated albumin-floxstop-Tag (AST) transgenic mice. The albumin promotor/enhancer and the SV40 Tag are separated by a stop-cassette flanked by loxP sites. After excision of the stop-cassette by cre recombinase, Tag oncogene expression is initiated.
AST mice crossed to cre-deleter mice, which express cre-recombinase ubiquitously, show Tag expression already two days after birth as a result of early embryonal cre recombinase activity. Tag expression results in the development of liver dysplasia at the age of four weeks, proceeding over nodular adenoma and carcinomata into multinodular HCC accompanied by a dramatically decreased life span of about 12 weeks. Tumor formation is observed exclusively in the liver, whereas AST single transgenic animals show no signs of tumor growth in the liver or other organs at the age of twelve months as examined by histology.
To obtain temporal control over Tag expression we generated recombinant adenoviral vectors encoding cre recombinase. Cre adenoviruses injected intravenously into AST mice mediate recombination in vivo. Somatic excision of the flox-stop cassette is monitored by analysis of genomic DNA and reveals recombination activity mainly in the liver due to the natural tropism of the adenovirus. Within three months Cre adenovirus-treated animals develop liver tumors in a dose dependent manner. Low dose adenovirus injection leads to the formation of dysplasia and small nodular adenoma or carcinoma while high dose inoculation results in multinodular HCC.
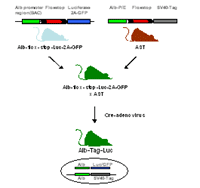
To be able to monitor tumor growth in vivo we are generating new inducible reporter mice expressing luciferase and the green fluorescent protein (GFP) upon cre-mediated recombination (Fig. 1). The coding sequences for luciferase and GFP are connected via a 2A sequence, which leads to the bi-cistronic expression of two independent proteins from one promoter (Szymczak et al., 2004). This allows the simultaneous detection of both luciferase-catalyzed bioluminescence and GFP-emitted fluorescence in one cell.
The beta-actin-flox-stop-Luc-2A-GFP mouse provides a versatile tool due to the ubiquitous expression of the reporter genes and can be crossed to different inducible oncogene transgenic mice. The Albumin-flox-stop-Luc-2A-GFP mouse was generated using the BAC (bacterial artificial chromosome) technology, which ensures a faithful expression of the reporter genes in the liver. Both reporter mice can be crossed to the AST mice. Double transgenic offspring will be injected with Cre-adenovirus to initiate the expression of both the oncogene Tag and the reporter genes luciferase and GFP. This allows the detection of tumor nodules in vivo by measuring the bioluminescence and/or fluorescence.
The crossing of different transgenic mice is time consuming and laborious. To circumvent these problems we are generating Albumin-floxstop-Tag-2A-Luc mice. Upon injection of Cre-adenovirus the successful recombination ensures the expression of both the oncogene Tag and the reporter gene luciferase.
Monitoring tumor growth by in vivo bioluminescence
To target tumors in vivo not only reporter mice but also luminescent bacteria can be used. These bacteria express luciferase and produce their own substrate as well so that the addition of an exogenous substrate is not necessary. When injected intravenously into mice these bacteria enter and selectively expand within solid tumors (Yu et al., 2004).
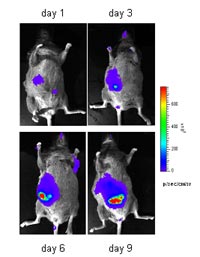
By in vivo bioluminescence we can monitor the growth of transplantation and autochthonous tumors in Albumin-Tag mice, which develop HCC spontaneously (Fig. 2, in collaboration with A. Szalay, Würzburg)). After intravenous injection the luminescent bacteria accumulate in the liver. The bioluminescence signal increases over time indicating the expansion of bacteria in the growing tumor nodules.
This approach is also suitable for the detection of liver tumors induced in AST mice. Currently, we are investigating the correlation between the detected bioluminescent signal and the tumor burden or the number of tumor nodules in Cre-adenovirus treated AST mice.
Outlook
Different antitumor therapies have been described to be promising in animal models. However, translation of these encouraging results into the clinic is difficult and only of limited success. This may be partly due to the use of transplantation tumors for the development of therapies. Autochthonous tumor models with inducible oncogene expression provide advanced and sophisticated systems to come as close as possible to the clinical situation of sporadic cancer formation.
The inducible AST mouse represents such a physiological system for sporadic tumors. It allows to elucidate tumor development from a few transformed cells in the autochthonous environment and to investigate mechanisms of tolerance induction towards tumors. This work is supported by the establishment of further tumor mice like a model for breast cancer. Future effort will focus on the evaluation of therapeutic or vaccination strategies. The newly generated luciferase reporter mice provide useful tools for the monitoring of these therapeutic approaches.
Lit.: 1. Hanahan D. et al., Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985 May 9-15; 315(6015):115-22. 2. Ganss R. and Hanahan D., Tumor microenvironment can restrict the effectiveness of activated antitumor lymphocytes. Cancer Res. 1998 Oct 15; 58(20): 4673-81. 3. Garbi N. et al., CpG motifs as proinflammatory factors render autochthonous tumors permissive for infiltration and destruction. J Immunol. 2004 May 15;172(10):5861-9. 4. Szymczak AL et al., Correction of multi-gene deficiency in vivo using a single 'self-cleaving' 2A peptide-based retroviral vector. Nat Biotechnol. 2004 May;22(5):589-94. 5. Yu YA et al., Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004 Mar;22(3):313-20.


