Introduction
Malignant gliomas, the most frequent primary tumors of the brain, are among the cancer diseases in man with the worst prognosis. This poor clinical course is paralleled by a high incidence of chromosomal imbalances in malignant gliomas causing the activation of oncogenes and the inactivation of tumor-suppressor-genes. Most of the relevant target genes, however, which influence tumorigenesis and progression in these tumors are still unknown.
The techniques recently used for the identification of cancer relevant genes include linkage analysis and positional cloning in patients with cancer predisposition diseases (1) and positional cloning of translocation breakpoints in tumor chromosomes (2, 3). By the latter method two novel genes, i.e. LGI1 (2) and WDR11 (3), were identified to be rearranged and downregulated in human malignant gliomas.
Cytogenetic analyses show that malignant gliomas contain complex alterations. Numerical chromosomal aberrations and marker chromosomes, which correspond to derivative chromosomes containing translocated material, can be identified by conventional cytogenetic banding techniques. However, it is necessary to apply molecular cytogenetic approaches, such as mFISH (4), SKY (5), CGH (6) and/or mCGH (7), to unequivocally identify the chromosomal regions contained in marker chromosomes, e.g. the translocated material. We are thus applying mFISH and CGH together with conventional banding to comprehensively identify recurrent chromosomal translocations in malignant glioma cell lines. To further characterize selected chromosome translocations, we will perform FISH experiments with BAC / PAC probes mapping to the respective chromosomal regions in order to identify breakpoint spanning probes. Positional cloning of the translocation breakpoint will then allow the identification of novel genes rearranged in malignant glioma cells.
Results/Project Status
The milestone we planned to reach in the 1st year of the project was the identification of relevant chromosomal translocations in malignant glioma cell lines. To obtain these results, chromosome preparations were generated and DNA was extracted from malignant glioma cell lines. A karyotype was derived from cell lines LN-428 (de novo glioblastoma from a 48-year-old male patient), LN-18 (de novo glioblastoma from a 65-year-old male patient), U251MG (de novo glioblastoma from a 75-year-old male patient), LN-229 (de novo glioblastoma from a 60-year-old female patient) and LN-319 (de novo anaplastic astrocytoma from a 67-year-old male patient) by combined analysis of conventional cytogenetics by GTG-banding and molecular cytogenetics by 24-color-FISH-analysis. A screen for genomic imbalances was performed for LN-428, U251MG, U373MG, LN-229, and LN-319 by chromosomal CGH (CGH) and array-based CGH (aCGH).
The comprehensive cytogenetic and molecular cytogenetic characterization of glioblastoma cell line LN-428 is given as an example in the following figures and text.
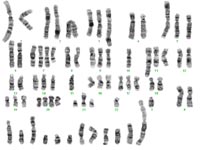
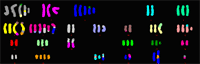

The composite karyotype derived from 17 metaphases obtained from glioblastoma cell line LN-428 was: 55~76,X,der(X)t(X;20)t(X;20),der(Y)t(Y;18),+der(?1),
der(1)t(1;22),der(1)t(1;19)t(1;21),der(3)t(1;3),der(3)t(3;21),-4, der(5)t(5;10),+der(6)t(6;10)t(6;15),+der(6)t(6;10)t(1;6)t(1;20), der(7)t(7;18),der(7)t(7;19),der(7)t(7;19)t(3;19),-8,der(9)t(8;9), der(9)t(9;17),-10,der(?10),der(11)t(11;15),der(15)t(7;15), der(15)t(8;15),+der(?17),der(18)t(17;18),der(19)t(3;19),+20, der(22)(?)[17cp].
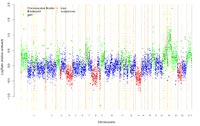
The imbalances detected by chromosomal CGH were: rev ish enh(3p21–p26,7p22–q22,9p21–q34,17p,17q25,19,20,X, Y); dim(4p13–q35,8p23–q22,9p21–p24,13q).
The results of array-based CGH-analysis on a genomic DNA-chip containing 8.000 large insert clones (kindly provided by B. Radlwimmer and P. Lichter, German Cancer Research Center, Heidelberg) was: enh(1p35.2–p36.3, 1p22.3–p31.3,1p13.1–p13.3,1q21.1–q23.1,1q32.1–q32.2,1q41–q44,2q35,2q37.2–q37.3,3p14.3–p26.3,5p15.3,6p12.3–p21.32,6q27,7p,7q21.3–q22.1,9p21.3–q34.3,10p15.1–p15.3,10q26.3,11p15.5,11q12.1–q13.5,11q23.1–q23.3,12p13.31–12p13.33,12q13.11–q13.3,14q32.32–q32.33,16p13.3,16q24.3,17p,17q25.1–q25.3,18q21.2–q23,19,20,X,Y),dim(1p36.3,1q32.2–q41,3p14.3–q11.2,4p16.1–q35.2,8p23.3–q23.3,8q24.3,9p21.3–p24.3,12q23.2,13q,22q11.21–q12.3),amp(19p13.11,19q13.12-q13.2).
The other malignant glioma cell lines investigated were characterized similarly. The main aim of this comprehensive analysis was to identify recurrent chromosomal translocations for further molecular breakpoint characterization. These were found to be translocation t(1;19) in LN-251 and LN-428, t(1;20) in LN-229 and LN-428, t(3;16) in LN-18, LN-229 and LN-251, t(3;19) in LN-229 and LN-428, t(4;6) in LN-229 and LN-251, t(5;15) in LN-18 and LN-229, t(6;8) in LN-229 and LN-319, t(6;19) in LN-18 and LN-319, t(8;14) in LN-18 and LN-229 as well as t(11;15) in LN-251 and LN-428.
Outlook
As a basis for the selection of pertinent chromosomal translocations for molecular analysis, the translocations identified recurrently in malignant glioma cells to date will be characterized further by cytogenetic techniques. This will involve the identification of the chromosomal subbands that the breakpoints are located in. In addition, a comprehensive analysis of structural and numerical aberrations will be performed in more malignant glioma cell lines. These data will allow the identification of the most relevant translocations in malignant gliomas, i.e. the translocations that are most frequent or that involve chromosomal regions known to be commonly altered in malignant gliomas.
As a next step, the characterization of the relevant chromosomal translocation breakpoints will be performed on a molecular level. This will be done by FISH analyses with BAC / PAC clones selected on the basis of information available from human genome databases. These experiments will allow to identify BAC / PAC clones that cross the breakpoints. Bioinformatic analyses of breakpoint spanning clones using databases such as ENSEMBL and NCBI will be performed. The strategy subsequently used will depend on the known and predicted gene content of the clones. It may be necessary to refine mapping by building a cosmid map of the region. Finally, the DNA from the respective malignant glioma cell line will be analyzed for rearrangements in the identified candidate genes.
Tumor-relevant genes may also be contained in small deletions or duplications sometimes found at chromosomal translocation breakpoints. Such microimbalances can be detected by array-based CGH. Genome databases give information about the known genes located in the deleted or duplicated chromosomal regions so that target-genes can be identified.
Candidate genes found to be rearranged, deleted or duplicated/amplified in glioma cell lines in these experiments will be subjected to molecular analyses in a large series of primary gliomas including cases of each major glioma entity (diffuse astrocytoma, anaplastic astrocytoma, primary glioblastoma, secondary glioblastoma, oligodendroglioma, anaplastic oligodendroglioma, oligoastrocytoma, anaplastic oligoastrocytoma). In the case of tumor suppressor genes, biallelic inactivation will be analyzed by mutation analyses and loss of heterozygosity studies. Possible inactivation by hypermethylation and/or transcriptional downregulation will have been determined in the subproject of Waha and Reifenberger using differential methylation hybridization (DMH) and microarray-based mRNA expression profiling. In the case of oncogenes, activation by amplification and/or transcriptional overexpression will be analyzed in primary gliomas.
This approach will reveal new genes associated with malignant gliomas. Providing new insights into the molecular basis of tumor formation, our results will have an impact on tumor classification as well as prediction of prognosis or response to therapy.
Lit.: 1. Liaw D et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997 May;16(1):64-7. 2. Chernova OB et al. A novel gene, LGI1, from 10q24 is rearranged and downregulated in malignant brain tumors. Oncogene. 1998 Dec 3;17(22):2873-81. 3. Chernova OB et al. A novel member of the WD-repeat gene family, WDR11, maps to the 10q26 region and is disrupted by a chromosome translocation in human glioblastoma cells. Oncogene. 2001 Aug 30;20(38):5378-92. 4. Speicher MR et al. Karyotyping human chromosomes by combinatorial multi-fluor FISH. Nat Genet. 1996 Apr;12(4):368-75. 5. Schrock E et al. Multicolor spectral karyotyping of human chromosomes. Science. 1996 Jul 26;273(5274):494-7. 6. Kallioniemi A et al. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science. 1992 Oct 30;258(5083):818-21. 7. Solinas-Toldo S et al. Matrix-based comparative genomic hybridization: biochips to screen for genomic imbalances. Genes Chromosomes Cancer. 1997 Dec;20(4):399-407.


