Introduction
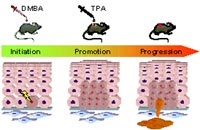
For the past half century, mouse skin carcinogenesis has been an important tool for developing the current concepts regarding human neoplasia and the multistage nature of tumour development. One of the best-defined and most extensively utilized experimental in vivo system for epithelial malignancy is the chemically induced tumour model of mouse back skin [1, 2]. This model is based on an initiation step due to a single treatment of mouse back skin with the mutagen 7, 12-dimethylbenz[a]anthracene (DMBA) leading to irreversible DNA damage. Subsequent tumour promotion through repeated applications of 12-O-tetradecanoylphorbol-13-acetate (TPA) results in the formation of multiple, benign papillomas some of which spontaneously progress to malignant, invasively growing squamous cell carcinomas (SCCs). It is well established that different types of genetic alterations can occur during multistage carcinogenesis and that these variations together with epigenetic changes are the main cause for differential expression of tumour-associated genes. Recent studies in genetically modified cell culture and mouse models revealed that an increased transcriptional activity of the transcription factor AP-1 is required for tumor promoter-induced transformation of mouse keratinocytes and is implicated in the conversion of benign to malignant tumours in the course of skin carcinogenesis [3]. AP-1 collectively describes a group of structurally and functionally related members of the Jun (c-Jun, JunB and JunD), Fos (c-Fos, FosB, Fra-1 and Fra-2) and ATF (ATFa, ATF-2 and ATF-3) protein families that bind as homo- and heterodimers to TPA-responsive elements in the promoter regions of target genes [4, 5]. AP-1 mediates gene regulation in response to a plethora of physiological and pathological stimuli and is implicated in various cellular processes, such as proliferation, differentiation, apoptosis and transformation [6, 7].
Results/Project Status
Identification of Novel Tumour-associated Genes During Multistage Carcinogenesis
We combined the chemically induced tumour model with comprehensive gene expression analysis and could identify a large set of novel tumour-associated genes that have not been associated with epithelial skin cancer development yet [8-12]. Self-organizing map clustering was performed to identify different kinetics of gene expression and co-regulation during skin cancer progression. Moreover, detailed analysis of differential expressed genes according to their functional annotation confirmed the involvement of several biological processes, such as regulation of cell cycle, apoptosis, extracellular proteolysis and cell adhesion, during skin malignancy [11]. In line with the important role of AP-1 in epithelial transformation, some of the differentially expressed genes are known AP-1 target genes or exhibit potential AP-1 binding motifs within their promoter region [9, 12-14]. As an example, we could show that the small Ras-related GTPase Rab11a is a novel, tumour-associated c-Fos/AP-1 target gene expressed in SCCs of mouse and human [9]. Since Rab11a is a major regulator of intracellular protein transport, our data link alterations in the cellular trafficking machinery mediated by AP-1 activity to the process of carcinogenesis.
Identification and Characterization of a novel TPA-inducible Aspartic Proteinase-like Gene
In addition to well-characterized genes, our screen also resulted in the identification of several tumour-associated EST and Riken clones coding for hitherto unknown proteins. Thus, we could clone a TPA-inducible cDNA from mouse back skin that encodes a novel aspartic proteinase-like protein, which we called Taps [13]. Interestingly, we observed during chemically induced mouse skin carcinogenesis a transient elevation of Taps in benign tumours, whereas expression was negatively correlated with dedifferentiation and malignant progression. Similar expression was detected in squamous skin carcinomas of human patients suggesting that detection of Taps levels represent a novel strategy to discriminate the progression state of epithelial cancers.
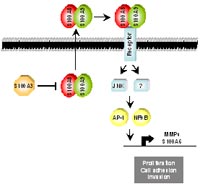
Antagonistic Function of S100 proteins regulates AP-1 and NF#B dependent gene regulation
Our global expression analysis elucidated differential expression of several members of the S100 protein family, located on human chromosome 1q21, during skin carcinogenesis. S100 proteins are low molecular weight proteins that exist as anti-parallel hetero- and homodimers. Upon calcium binding they can interact with target proteins to regulate cellular processes, such as cytoskeleton dynamics, cell signalling, cell differentiation, cell cycle progression and metastatic transformation [15]. Despite compelling data demonstrating a direct link between altered expression of S100 proteins and common human malignancies, including human prostate cancer [18], the knowledge of their function and mode of action in epithelial cells and in the progress of cancer is unknown. We have identified a novel S100A8/A9 signalling pathway in epithelial cells resulting in the activation of AP-1 and NF#B [14]. Importantly, another member of the S100 family, S100A3, whose expression is down-regulated during chemically induced skin carcinogenesis in mice, antagonizes S100A8/A9 signalling activity, suggesting a critical role of S100A3 in cancer development. Finally, we could identify S100A6 as a target gene of the S100A8/A9 signalling pathway and found that elevated expression of S100A6 induces cell growth of mouse keratinocytes and correlates with epithelial malignancy in humans. Our data suggest that targeting the net activity of S100-induced signalling represents a novel strategy for innovative cancer prevention and therapy.
Outlook
Our comprehensive list of tumour-associated genes represents a valuable source to elucidate crucial mediators of neoplastic transformation and to understand the molecular principles underlying the multistage nature of epithelial cancer, but also other malignancies. Since, recent publications clearly demonstrated that alterations in AP-1 activity and in AP-1 target gene expression is critical for development of brain tumours [16, 17], we are convinced that some of the differentially expressed genes, identified in skin carcinogenesis, may also contribute to neoplastic transformation of neuronal cells. Therefore, we initiated a comparison of our data with gene expression studies performed on human brain tumours and investigate expression of common candidate genes on tumour specimens. Additionally, we currently establish gain-of-function and loss-of-function approaches using genetically modified cell culture and mouse models to get insight into their functional role in the development and progression of tumours.
Lit.: 1. Marks, F., and Furstenberger, G. (1990). The conversion stage of skin carcinogenesis. Carcinogenesis 11, 2085-2092. 2. Yuspa, S.H. (1998). The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis. J Dermatol Sci 17, 1-7. 3. Young, M.R., Yang, H.S., and Colburn, N.H. (2003). Promising molecular targets for cancer prevention: AP-1, NF-kappa B and Pdcd4. Trends Mol Med 9, 36-41. 4. Angel, P., and Karin, M. (1991). The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta 1072, 129-157. 5. Wagner, E.F. (2001). AP-1 reviews. Oncogene 20, 2333-2497. 6. Eferl, R., and Wagner, E.F. (2003). AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 3, 859-868. 7. Hess, J., Angel, P., and Schorpp-Kistner, M. (2004). AP-1 subunits: quarrel and harmony among siblings. J Cell Sci 117, 5965-5973. 8. Breitenbach, U., Tuckermann, J.P., Gebhardt, C., Richter, K.H., Furstenberger, G., Christofori, G., and Angel, P. (2001). Keratinocyte-specific onset of serine protease BSSP expression in experimental carcinogenesis. J Invest Dermatol 117, 634-640. 9. Gebhardt, C., Breitenbach, U., Richter, K.H., Furstenberger, G., Mauch, C., Angel, P., and Hess, J. (2005). c-Fos-Dependent Induction of the Small Ras-Related GTPase Rab11a in Skin Carcinogenesis. Am J Pathol 167, 243-253. 10. Gebhardt, C., Breitenbach, U., Tuckermann, J.P., Dittrich, B.T., Richter, K.H., and Angel, P. (2002). Calgranulins S100A8 and S100A9 are negatively regulated by glucocorticoids in a c-Fos-dependent manner and overexpressed throughout skin carcinogenesis. Oncogene 21, 4266-4276. 11. Hummerich, L., Müller, R., Hess, J., Kokocinski, F., Hahn, M., Fürstenberger, G., Mauch, C., Lichter, P., and Angel, P. (in press). Identification of novel tumour-associated genes differentially expressed in the process of squamous cell cancer development. Oncogene. 12. Schlingemann, J., Hess, J., Wrobel, G., Breitenbach, U., Gebhardt, C., Steinlein, P., Kramer, H., Furstenberger, G., Hahn, M., Angel, P., and Lichter, P. (2003). Profile of gene expression induced by the tumour promotor TPA in murine epithelial cells. Int J Cancer 104, 699-708. 13. Rhiemeier, V., Breitenbach, U., Richter, K.H., Gebhardt, C., Fuerstenberger, G., Hess, J., and Angel, P. (submitted). Identification and characterization of a novel TPA-inducible aspartic protease-like gene expressed in stratified epithelia. Am J Pathol. 14. van der Sanden, M., Hummerich, L., Mueller, R., Gebhardt, C., Kokocinski, F., Tews, B., Hahn, M., Fuerstenberger, G., Mauch, C., Lichter, P., Angel, P., and Hess, J. (submitted). Antagonistic function of S100 proteins in the activation of NFkappaB and AP-1 dependent gene regulation during tumor development. J Cell Biol. 15. Donato, R. (2003). Intracellular and extracellular roles of S100 proteins. Microsc Res Tech 60, 540-551. 16. Assimakopoulou, M., and Varakis, J. (2001). AP-1 and heat shock protein 27 expression in human astrocytomas. J Cancer Res Clin Oncol 127, 727-732. 17. Debinski, W., Gibo, D., and Mintz, A. (2003). Epigenetics in high-grade astrocytomas: opportunities for prevention and detection of brain tumors. Ann N Y Acad Sci 983, 232-242. 18. Hermani, A., Hess, J., De Servi, B., Medunjanin, S., Grobholz, R., Trojan, L., Angel, P., and Mayer, D., (2005). Calcium-binding proteins S100A8 and S100A9 as novel diagnostic markers in human prostate cancer. Clin Cancer Res 11, 5146-52.


