Introduction
Alcoholism is affecting up to 5% of the population in industrial nations and thus has a major impact on the socio-economic system of our societies. Chronic ethanol abuse not only transiently affects cognitive behaviour but also often results in encephalopathy severely impairing the patient's health. Therefore, major efforts have been undertaken to identify the molecular mechanisms leading to alcohol dependence and tolerance.
The hypothalamic-pituitary-adrenocortical (HPA) axis is one of the biological systems which are affected by both stress and alcohol. The activity of the HPA system is regulated by the corticotropin-releasing hormone (CRH) and its cognate receptor the corticotropin-releasing hormone receptor type 1 (CRH-R1). It has been shown that administration of ethanol to rats rapidly upregulates CRH and CRH-R1 levels in the hypothalamus, followed by an upregulation of adrenocorticotropic hormone (ACTH) in the pituitary and of corticosterone released from the adrenal gland indicating that ethanol has an impact on hypothalamic stress hormone regulation. Moreover, the interactions of ethanol and the CRH system are not only restricted to hypothalamic regions. This notion is supported by animal studies that detected an increase of extracellular CRH in the amygdala during alcohol withdrawal. In addition, ethanol/CRH interactions within distinct brain regions, i.e. the extended amygdala, are hypothesized to be of motivational significance in the transition from nondependent to dependent drinking.
Recently, we could demonstrate that repeated physical stress caused by the forced swimming paradigm or psychological stress implemented via the social defeat paradigm leads to enhanced and progressively increasing alcohol intake. Interestingly, the effect of repeated stress on alcohol drinking behaviour appeared with a delay and persisted throughout the animals´ lifetime. Thus, CRH-R1 represents a genetic risk factor which, in combination with stress, induces enhanced alcohol drinking in animals. Nevertheless, it is not clear whether primarily an expression domain of CRH-R1 in the brain is responsible for enhanced alcohol drinking after stress exposure or whether secondarily corticosterone dysregulation underlies this effect.
To identify brain regions in which CRH-R1 is active and contributes to this specific phenotype we will generate somatic mutations of CRH-R1 taking advantage of the Cre/loxP system. We will specifically inactivate CRH-R1 in brain structures of the limbic system and in the HPA axis of mice, followed by the analysis of their alcohol drinking behaviour. These experiments will teach us whether the CRH-R1 effect on alcohol drinking behaviour is exerted via the limbic system or based on dysregulation of the HPA axis. After having shown which brain region is connected with CRH-R1 mediated alcohol drinking behaviour we will identify the genes downstream of CRH-R1 that mediate this effect using microarray technology followed by the validation of candidate genes in appropriate animal models.
Project Status
To differentiate CRH/CRH-R1-dependent neuronal circuitries in the central nervous system (CNS) that modulate behaviour from those that regulate neuroendocrine function via the HPA system, we are generating specific conditional CRH-R1 knockout mouse lines. The analysis of these conditional mutants will allow us to determine which expression domains of CRH-R1 in the brain influence alcohol drinking behaviour and to what extent HPA system dysregulation underlies the stress induced enhancement of alcohol drinking observed in CRH-R1 knockout mice.
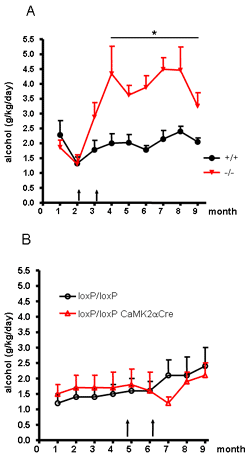
In CRH-R1loxP/loxP CaMKIIaCre mice CRH-R1 is inactivated postnatally in anterior forebrain and limbic brain structures, while sparing hypothalamic and pituitary expression sites and thus leaving HPA system regulation undisturbed. Additionally, major CRH-R1 expression domains in the brain stem (e.g. dorsal raphe nucleus and pontine gray) remain unaffected in these conditional mutants.
After a habituation period voluntary alcohol consumption of male conditional mutant (CRH-R1loxP/loxP CaMKIIaCre) and control (CRH-R1loxP/loxP) mice was monitored in a three-bottle free-choice paradigm (water, 4% or 8% alcohol). Conditional CRH-R1 knockout mice and control littermates did not differ in the intake or preference of alcohol when analysed under stress free housing conditions (Fig. 1).
Subsequently voluntary alcohol consumption was monitored during and after repeated stress. Stress episodes involved three consecutive days of social defeat stress as a severe psychological stressor followed by three consecutive days of forced swimming as a predominantly physical stressor. Conditional CRH-R1 knockout mice and control littermates did not differ in the intake or preference of alcohol when analysed during and immediately after the repeated stress episodes. In contrast to previous observations in conventional CRH-R1 knockout mice, conditional CRH-R1loxP/loxP CaMKIIaCre mutant mice did not show enhanced and progressively increasing alcohol intake following stressful experiences (Fig. 1). These results suggest that CRH-R1 in forebrain and limbic brain structures is not involved in acute or long-term effects of stress-induced alcohol drinking.
Outlook
In order to further narrow down CRH-R1 expression domains in the brain which might influence alcohol drinking behaviour we generated CRH-R1loxP/loxP NestinCre mice. In these conditional mutants CRH-R1 is inactivated during embryogenesis in the entire CNS including brain stem structures which were omitted in CRH-R1loxP/loxP CaMKIIaCre mice. Since the pituitary expression of CRH-R1 is not disrupted in CRH-R1loxP/loxP NestinCre mice, the HPA system will not be affected. Hence, this model will additionally shed light on the question whether primarily CRH-R1 in the brain is responsible for enhanced alcohol drinking after stress exposure or whether secondarily a HPA axis dysregulation underlies the observed effects.
As we could show, corticosterone release from the zona fasciculata of the adrenal cortex is regulated by CRH-R1. For this purpose we are establishing a transgenic mouse line in which Cre recombinase is driven by the ACTH receptor (ACTH-R) promoter. Crossing CRH-R1loxP/loxP mice with ACTH-R-Cre mice will enable us to determine whether altered corticosterone release is the underlying reason for enhanced post-stress alcohol drinking in CRH-R1 null mutants. Voluntary alcohol consumption of CRH-R1loxP/loxP NestinCre and CRH-R1loxP/loxP ACTH-R-Cre mice wil be studied as described for CRH-R1loxP/loxP CaMKIIaCre mice.
Finally, based on the outcome of the behavioural analysis of conditional mutants we will analyse those brain regions which are responsible for the enhanced alcohol drinking effect in comparison to respective wildtype controls by expression profiling.
Lit.: 1. Timpl P et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nature Genetics. 1998 June;19(2):162-6. 2. Sillaber I et al. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2003 May 3;296(5569):931-3. 3. Muller MB et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behaviour and hormonal adaption to stress. Naure Neuroscience. 2003 October;6(10):1100-7.


