Introduction:
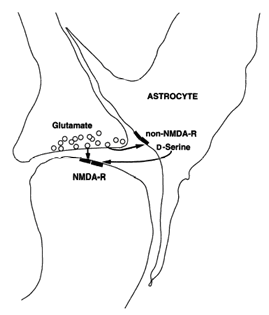
(Schell, Molliver et al. 1995).
We are currently generating mouse strains with decreased brain D-serine levels. Alterations of the D-serine concentrations in the brain should affect the activity of the NMDA receptor, which may result in a behavioral phenotype. Since the G72/G30 gene locus is not conserved in mice, we are introducing the human gene locus into the mouse genome via the BAC (Bacterial Artificial Chromosome) technology. Murine expression of DAOA could lead to a higher cerebral DAAO activity and thus lower cerebral D-serine levels. In addition to that, we will generate a transgenic mouse line with a glial overexpression of the murine DAAO protein, which again may have lower D-serine concentrations in the brain. Both mouse models should mimic the phenomenon of low D-serine levels measured in the brain of drug naïve schizophrenic patients (Hashimoto, Engberg et al. 2005). These mouse models will be analyzed in schizophrenia, depression and anxiety related behavioral tests.
Project status:
BAC-transgenic mice:
In order to generate and analyze transgenic mouse models with a human G72 gene locus, we selected two different BAC (Bacterial Artificial Chromosome) clones that cover that whole human G72/G30 gene region and ordered them from Children's Hospital Oakland Research Institute (CHORI) (RP11-111A8 and RP11-111A6). We have verified the identity of the BAC clones by PCR and Southern blot. A ca. 160 kb insert from BAC RP11-111A8 was released form the backbone by restriction, purified by gel electrophoresis and microinjected into fertilized eggs of CD1 mice. To this date we have identified four transgenic animals out of 53 offspring. The offspring of 2 of the four animals inherited the BAC-fragment and are currently analyzed for the expression of human transcripts.
DAAO overexpressing mice:
For expression of DAAO under the human GFAP promoter a ca. 1,6 kb EcoRI/NotI fragment, containing the whole DAAO-ORF from vector IRAKp961M024Q2 (RZPD-clone) was cloned into the EcoRI/NotI cut hGFAP pGEM T-easy vector (Barton, Dunlop et al. 2002) containing a ca. 2,2 kb fragment of the human GFAP promoter. The resulting vector was cut with EagI and AseI to release the GFAP promoter DAAO fragment. The fragment was injected in FVB/N oocytes. Currently offspring are analyzed for genomic fragment integration.
Outlook:
The aim of this project is the generation and analysis of transgenic mouse models with a human G72 gene locus and glial overexpression of the DAAO protein. These models will be used to study the expression of transcripts from this gene locus and to address its potential function in the modulation of neuronal signalling. The activity of NMDA receptors in the brain of transgenic animals will be investigated. Moreover both animal models are going to be analyzed in behavior tests related to depression, schizophrenia and anxiety
Lit.: 1. Addington, A. M., M. Gornick, et al. (2004). "Polymorphisms in the 13q33.2 gene G72/G30 are associated with childhood-onset schizophrenia and psychosis not otherwise specified." Biol Psychiatry 55(10): 976-80. 2. Barton, M. D., J. W. Dunlop, et al. (2002). "Modified GFAP promoter auto-regulates tet-activator expression for increased transactivation and reduced tTA-associated toxicity." Brain Res Mol Brain Res 101(1-2): 71-81. 3. Chen, Y. S., N. Akula, et al. (2004). "Findings in an independent sample support an association between bipolar affective disorder and the G72/G30 locus on chromosome 13q33." Mol Psychiatry 9(1): 87-92; image 5. 4. Chumakov, I., M. Blumenfeld, et al. (2002). "Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia." Proc Natl Acad Sci U S A 99(21): 13675-80. 5. Hashimoto, K., G. Engberg, et al. (2005). "Reduced D-serine to total serine ratio in the cerebrospinal fluid of drug naïve schizophrenic patients." Prog Neuropsychopharmacol Biol Psychiatry 29(5): 767-9. 6. Hattori, E., C. Liu, et al. (2003). "Polymorphisms at the G72/G30 gene locus, on 13q33, are associated with bipolar disorder in two independent pedigree series." Am J Hum Genet 72(5): 1131-40. 7. Korostishevsky, M., M. Kaganovich, et al. (2004). "Is the G72/G30 locus associated with schizophrenia? single nucleotide polymorphisms, haplotypes, and gene expression analysis." Biol Psychiatry 56(3): 169-76. 8. Law, A. J. and J. F. Deakin (2001). "Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses." Neuroreport 12(13): 2971-4. 9. Schell, M. J., M. E. Molliver, et al. (1995). "D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release." Proc Natl Acad Sci U S A 92(9): 3948-52. 10. Schumacher, J., R. A. Jamra, et al. (2004). "Examination of G72 and D-amino-acid oxidase as genetic risk factors for schizophrenia and bipolar affective disorder." Mol Psychiatry 9(2): 203-7. 11. Steinpresis, R. E. (1996). "The behavioral and neurochemical effects of phenyclidine in humans and animals: some implications for modeling psychosis." Behav Brain Res 74(1-2): 45-55. 12. Wang, X., G. He, et al. (2004). "Association of G72/G30 with schizophrenia in the Chinese population." Biochem Biophys Res Commun 319(4): 1281-6.


