Introduction
Aims of the project
Phenotype characterization of complex genetic disorders needs to extend beyond the mere establishment of dichotomous diagnoses (“Genes can’t read DSM-diagnoses!”). Detection of predisposition genes requires consideration of the influence of certain clinical and environmental features. Approaches that have applied such refined phenotype characterization strategies to date have yielded promising results (Caspi et al. 2003). The goal of this project is to establish a sample of 650 patients with bipolar affective disorder (BPAD) whose phenotype profile includes a highly detailed assessment of clinical, socio-demographic, and environmental factors. Phenotype characterization will also include advanced approaches such as structural and functional magnetic imaging (MRI) for a subgroup of patients. DNA from 300 of these patients is being used for the SNP-based LD mapping in project 8.3.1 (initial sample), and DNA from the remaining 350 patients is available for replication studies within this project (replication sample). Analyses of DNA from the full sample will enable us to formulate a model explaining the complex genetic susceptibility that underlies BPAD.
Background
The etiology of BPAD is multifactorial and is presumed to be based on several partly interacting genetic mechanisms which may, in addition, be modulated by environmental factors. It is anticipated that identification of these genes will reveal the underlying disease processes, something that is necessary for the development of improved diagnosis and treatment, and for the realization of the possibility of disease prevention.
There are two major approaches to the identification of vulnerability genes: association and linkage studies. In the past, association studies using the candidate gene approach (i.e. selection of genes on the basis of a presumed role in disease pathophysiology) have produced inconsistent results. This was attributable to limited insight into the pathophysiology of disorders, further aggravated by the wide host of potential candidates (all of the 20,000 genes expressed in the CNS being theoretical candidates). Association studies, focusing on candidate loci identified through linkage studies, are expected to deliver more consistent results in future research. A variety of genome-wide linkage scans for BPAD have recently been completed. Overlapping results have been reported for the following chromosomal loci: 1p, 4p, 4q, 6q, 8q, 10q, 12q, 13q, 18p, 18q, 22q. These chromosomal region are still very broad, however, spanning regions of up to 50 centiMorgans (cM). Decreasing the heterogeneity of BPAD by dividing its phenotype into meaningful subtypes will enhance the identification of susceptibility genes (Lander & Schork 1994), as has been shown by studies of other genetically complex disorders. Such a systematic approach has yet to be pursued in BPAD, and appropriately large and well-characterized samples will be an indispensable prerequisite for its introduction.
We have established one of the largest samples world-wide for use in linkage and association studies, comprised of 620 bipolar patients and 1,195 population-based controls, all of German descent. In addition, we have established family and case-control samples within four other European populations (Andalusia/Spain, Russia, Poland, former Yugoslavia), totalling 717 patients and 519 controls.
We have established linkage to several chromosomal regions in the German and Andalusian samples. The size of our German case-control sample provides sufficient power for the replication of most association findings. Using our German sample, we have been able to replicate the reported association of BPAD with G72 (Schumacher et al. 2004), the first gene for schizophrenia to be identified through positional cloning . Furthermore, we have found that this association is driven by those BPAD patients with a life-time history of persecutory delusions. We found no overall association between BPAD and G72 in the Polish sample. However, there was a significant association when case definition was restricted to only those BPAD patients with a history of persecutory delusions (Schulze et al., in press). Thus, for the first time in the history of psychiatric research, it was possible to prove the existence of an overlap between schizophrenia and BPAD at the molecular genetic level. This use of clinically defined case-definitions that extend beyond traditional categorical diagnoses will be a key future strategy for the identification of genetically homogeneous phenotypes since, in psychiatric disorders, the observed phenotype is best viewed as a syndrome rather than a disease entity. It is uncertain which of the distinct phenotypic features defines the genetically determined ‘core’ phenotype. Given the presumed etiological heterogeneity of BPAD, for which no biologically meaningful diagnostic tests exist, the conducting of studies using diverse proportions of “subtypes” may profoundly affect genetic findings.
A reduction in phenotypic heterogeneity in research samples may increase genetic homogeneity, and thereby enhance the likelihood of identifying vulnerability genes for BPAD. This approach requires readily available and comprehensive phenotype information. In our sample of 620 patients such information (including environmental factors) has been collected for 336 patients. In our other patient samples from Poland, Russia, Andalusia, and the former Yugoslavia, we have applied this comprehensive phenotype characterization already.
Characteristics of the project
Our phenotype characterization had focused initially on establishing valid life-time categorical diagnoses according to ICD, RDC and DSM criteria. We have subsequently extended our phenotype characterization to include consideration of life-time symptoms, detailed course of the disorder, pharmacoresponse, information on premorbid adjustment, stressful life events and environmental factors such as birth complications and parental bonding. We have established a relational database detailing more than 2,000 items per person (Fangerau et al. 2004). Our current sample is comprised of 620 individuals with BPAD. We aim to achieve a final sample size of 650 patients for whom the full complement of the aforementioned items will be available. This level of detailed phenotype information is available for 336 patients currently. To achieve our goal we are in the process of re-assessing 284 participants from previous studies, and are continuing to recruit new BPAD patients from consecutive admissions to our clinical centers. Continuous fresh recruitment is indispensable, since we cannot rely on including of all of the 284 previous study participants for further assessment. The availability of such a large sample of well-characterized patients provides improved statistical power for conditional or covariate-based linkage and association studies. The detailed components of this strategy are as follows:
(1) We are re-contacting patients who have previously participated in our BPAD study in order to complete our comprehensive phenotype characterization on them. (2) Continuous recruitment of patients from consecutive admissions to our clinical centers is in progress to ensure recruitment of the target sample of 650 patients. Whenever possible, we incorporate neuro-imaging into the phenotype characterization of patients. Blood samples are taken from all patients. (3) Both clinical and genetic data are managed centrally. Our site is responsible for data transfer between the clinical, molecular- genetic and statistical centers. Our site oversees the consistency of phenotype characterization and documentation across the various centers. Data management falls into three broad categories: a) clinical data of a sensitive nature which includes personal identification; b) anonymized clinical data; and c) data generated by molecular methods in the laboratory, including genotypes, sequences etc. These elements of data management occur within this project. Subsequent systematic fine-mapping and association analyses are performed in collaboration with the GEM platforms.
Our samples from patients with BPAD are analyzed additionally to define the biological relevance of disease genes that have been identified in patients with unipolar depressive disorder (UPD) by project 8.3.3. Since UPD and BPAD are associated strongly within families, it is of particular interest to formulate a model for the complex genetic susceptibility underlying affective disorder using our highly characterized patient samples.
Results/Project Status
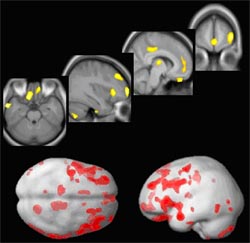
Since the beginning of the project, we have recruited and phenotyped 87 patients with BPAD using the full spectrum of diagnostic assessment tools mentioned above. In addition, we have introduced a more sophisticated phenotype characterization approach, i.e. the use of structural MRI as outlined below: The occurrence of both depressive and manic episodes, intercepted by euthymic (i.e. normal mood) intervals, is the key feature of BPAD. Euthymic intervals in a patient suffering from BPAD are considered to be characterized by recovery from impairment in areas of everyday functioning. However, recent findings suggest the persistence of neuropsychological deficits (e.g. in working memory, fluency of speech, episodic memory) during such intervals of remission from affective disturbance. This may be due to a persistant prefrontal dysfunction. To assess potential disease correlates in brain morphology, we used MRI to investigate a sample of 42 patients with BPAD and 42 sex- and age-matched healthy controls (1.5 Tesla). Patients showed a significant reduction in grey matter volume, particularly in prefrontal and temporal regions of the brain (see Figure 1). This reduction was not limited to the “dorsal” areas of the cortex which are involved in higher cognitive processes (e.g. dorsolateral-prefrontal cortex, mediodorsal thalamus, anterior insula, posterior-prefrontal cortex) but also “ventral” areas, which are known to be associated with the regulation of emotion (e.g. orbito-frontal cortex, ventral aspects of the medio-frontal cortex, anterior gyrus cinguli). We also found a significant increase in cerebrospinal fluid in the respective neighbouring cavities (e.g. frontal interhemispheric fissure, Sylvian fissure, third ventricle) a finding which is consistent with an underlying atrophic process (Tost et al. 2005).
Outlook
Within the framework of this project, we will establish a comprehensively characterized sample of 650 patients with BPAD, which will facilitate gene mapping efforts for this disorder. Our extensive phenotypic data will be used to study complex genotype-phenotype correlations. We are now broadening our phenotype characterization to include endophenotypes (i.e. structural and functional MRI), which are believed to show a less complex relationship to the underlying genetic basis than are the psychiatric diagnostic categories (Schulze & McMahon 2004). Subgroups of BPAD patients with specific features of brain morphology and/or function may prove to be a valuable tool for better understanding and dissecting the heterogeneity of the disorder.
Lit.: 1. Caspi A al.(2003) Science 301:386-389 2. Lander ES & Schork NJ (1994) Science 265: 2037-2048. 3. Schumacher J et al. (2004) Mol Psychiatry 9: 203-20 4. Schulze TG et al. (2005) Am J Psychiatry, in press 5. Fangerau H et al. (2004) Hum Heredity 58: 122-130 6. Tost H et al. (2005), Annual Meeting of the Organization for Human Brain Mapping (Toronto,June 12-16, 2005) 7. Schulze TG & McMhaon FJ. (2004) Hum Heredity 58: 131-138


