Introduction
Neural stem cells exist in the adult human mammalian brain throughout the entire lifetime. These cells are capable of self-renewal, proliferation, and differentiation into brain cells, most predominantly neurons and astrocytes (1). Neural stem cells in the adult mammalian brain are restricted to defined areas of ongoing neurogenesis, including the dentate gyrus of the hippocampus and the subventricular zone. From the latter, neural stem cells migrate tangentially along the rostral migratory stream towards the olfactory bulb and differentiate. Radial migration from the subventricular zone to the cerebral cortex seems to be limited to embryonic developmental stages.
Neural stem cells and more determined neural progenitor, or precursor cells, can be dissected from distinct brain regions and kept in cell culture. This in vitro expansion known as neurosphere assay, may allow generating a sufficient number of neural stem cells which can be used for subsequent transplantation. These new therapies based on the replacement of damaged tissue after stroke with the goal to re-establish lost function consist of either activation of endogenous stem cells or transplantation of stem cells from an exogenous source. For any kind of therapy, neural stem cells, whether of endogenous or exogenous source, will need an alignment of their genetic program to survive, proliferate and differentiate within the brain. For these functions, growth factors play an essential role in the brain, among them the basic Fibroblast Growth Factor, Endothelial Growth Factor, Bone Morphogenetic Proteins, Sonic Hedgehog Signaling, and many others.
Besides these growth factors, erythropoietin appears to be one of the major players in this context. Erythropoietin is a 34 kDa glycoprotein primarily produced in the adult kidney upon hypoxic stimuli. It is the principal growth factor regulating red blood cell production (2). Several studies showed that erythropoietin is an important survival factor for neurons under hypoxic-ischemic conditions in vivo and in vitro (3, 4). During brain development, erythropoietin acts as neurotrophin and has anti-apoptotic effects under the hypoxic-ischemic conditions. In the adult, erythropoietin may also serve as neuroprotective factor in stroke (5). With regard to neural stem cells, erythropoietin directly promotes the generation of neuronal stem cells from progenitors, and induces migratory behavior (6).
The current project in the National Genome Research Network consists of 4 subsequent steps of experiments in adult neural stem cells which have the following goals:
1. To trace cellular and molecular pathways which are influenced by erythropoietin. We isolate adult neural stem cells from the brain of 3-6 week old rats and analyze the changes in the proteome of the cultured neural stem cells when exposed to erythropoietin. Proteomics is a mass-screening approach for the simultaneous expression analysis of hundreds, or even thousands of proteins. We use an approach based on two-dimensional gel electrophoresis for protein separation, and mass spectrometry for protein identification.
2. To verify the biological function of the identified cellular and molecular pathways activated by erythropoietin in the neural stem cells with regard to their differentiation potential and migration behavior. To this end, specific blocking reagents are applied and the tissue is analyzed by immunohistochemical methods.
3. To detect the influence of erythropoietin on the proteome and the survival of neural stem cells during hypoxic conditions, especially to find protective mechanisms of erythropoietin during stem cell hypoxia. In these experiments, neural stem cells are exposed to severe hypoxic conditions in an incubation chamber (Fig. 1) for defined periods of time, subsequently reoxygenized and subjected to proteome analysis.
4. To analyze the outcome of an experimental stroke induced in rats and mice after transplantation of genetically modified neural stem cells into their brains. To this end, neural stem cells are transfected with vectors containing the erythropoietin or erythropoietin receptor gene and are then stereotactically transplanted into the brain.
Results/Project Status
In the first part of the project, we concentrated on the effects of hypoxia on neural stem cells in vitro and tried to elucidate the role of erythropoietin in cell culture experiments on neural stem cell survival after hypoxia. Hypoxic effects have been well investigated with regard to neurons, or the brain in general, but they are not clearly defined in neural stem cells. We cultured neurospheres from hippocampus, olfactory bulb and subventricular zone of adult rat brains and exposed the cells to erythropoietin levels from 0.01 to 100 units/ml media either in normoxia or in anoxia in a humidified gas mixture of 5% CO2 under a N2 atmosphere for 24 hours (Fig 1).

Cell viability and apoptosis rates were determined biochemically. We found that all three neural stem cell lines showed increased proliferation during anoxia, but with significant heterogeneities in their growth and proliferation behavior depending on their regional provenience.
Proteomic analysis
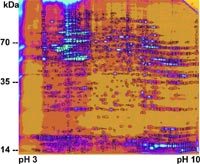
In two-dimensional gels, we compared global protein expression patterns of neural stem cells which have been incubated in a dose-dependent manner with erythropoietin (Fig 2). We also isolated proteins from neural stem cells after hypoxia in vitro and separated these proteins by two-dimensional electrophoresis. The results were compared to proteomic reference maps for neural stem cell proteins (7, 8). We found significant changes in the protein expression patterns, both in the cells stimulated by erythropoietin as well as in the hypoxic cells. Moreover, reoxygenation may increase cellular damage even in early stages after the end of hypoxia.
We are now analyzing biochemical pathways involved in signal transduction of the erythropoietin effect on neural stem cells using bioinformatic tools (10), reporter gene assays and the assessment of biochemical properties. We are also analyzing erythropoietin signaling pathways using chemically modified erythropoietin molecules and analyzing their receptors and targets in the brain (11, 12).
Outlook
After completion of the first part of the project, we will investigate the effect of erythropoietin on neural stem cells in vivo. Erythropoietin is considered as a clinically applied therapeutics in a variety of diseases, but its effects on neural stem cells is still not well defined. In the light of our recent findings, we will continue to elucidate a role of erythropoietin on neural stem cells in an integrative approach both in vitro and in vivo. We will focus on experimental stroke models as paradigm for neurological disease in which stem cells can become a therapeutical means.
Lit.: 1. Maurer MH and Kuschinsky W. Screening the brain: molecular fingerprints of neural stem cells. Curr Stem Cell Res Ther. 2006:in press. 2. Jelkmann W. Erythropoietin: structure, control of production, and function. Physiol Rev. 1992;72(2):449-89. 3. Marti HH. Erythropoietin and the hypoxic brain. J Exp Biol. 2004 Aug 15;207(Pt 18):3233-42. 4. Jelkmann W. Effects of erythropoietin on brain function. Curr Pharm Biotechnol. 2005 Feb;6(1):65-79. 5. Brines M and Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005 Jun;6 (6):484-94. 6. Shingo T et al. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21(24):9733-43. 7. Maurer MH et al. Comprehensive proteome expression profiling of undifferentiated vs. differentiated neural stem cells from adult rat hippocampus. Neurochem Res. 2004 June;29(6):1129-44. 8. Maurer MH et al. The proteome of neural stem cells from adult rat hippocampus. Proteome Sci. 2003;1(1):4. 9. Maurer MH and Kuschinsky W Proteomics. In: Handbook of Neurochemistry and Molecular Neurobiology, 3rd ed. Lajtha A, ed. Springer:New York, in press. 10. Maurer MH. The path to enlightenment: making sense of genomic and proteomic information. Genomics Proteomics Bioinformatics. 2004 May;2(2):123-31. 11. Leist M et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004 Jul 9;305(5681):239-42. 12. Brines M et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci U S A. 2004 Oct 12;101(41):14907-12.


