Introduction
Improving care and treatment of stroke includes both development of new pharmacological strategies in the acute phase(Fisher and Schaebitz, 2000; Schabitz et al., 2003), as well as improvements in approaches that stimulate functional recovery after stroke such as physical therapy (Nudo et al., 2001). Immobilization of the unaffected arm combined with physical therapy, the so called forced use paradigm, was shown to improve motor function of the impaired arm weeks after unilateral stroke humans (Miltner et al., 1999). However, experimental studies are controversial, and indicated that early forced motor training of the impaired limb worsened functional outcome and exaggerated lesion size after cerebral ischemia (Kozlowski et al., 1996; Risedal et al., 1999). Large lesions of the cortex include larger penumbral areas with cells at risk where behavioural pressure may further increase energy failure and finally kill these cells. Small lesions have no or smaller penumbral areas with fewer cells at risk so that behavioural pressure could be compensated by living cells which may then improve function through early training (Bland et al., 2001, Schäbitz et al. 2004). Forced motor training was also reported to strongly activate the hypophysiotropic CRH system, which indicates a strong stress component of forced treatment paradigms (Timofeeva et al., 2003). These findings together raise questions about early and intensive rehabilitation programs after stroke in humans. Voluntary running is probably the most natural behavior of rodents. Recently it was shown that voluntary wheel running as model for enriched environment improved hippocampal neuroplasticity (Molteni et al., 2002). Voluntary treatment paradigms were also shown to improve recovery and to induce plasticity after focal cerebral ischemia (Dahlqvist et al., 2003). The genomic mechanisms involved in plasticity changes after different means of rehabilitative training poststroke are largely unexplored. Several gene expression studies after cerebral ischemia describe a number of regulated genes and gene families in relation to the acute phase of the ischemic event. However, knowledge of genes involved in longterm and rehabilitation-mediated plastic changes is limited. The clarification of these changes would potentially allow a pharmacological targeting of of mechanisms of rehabilitation-induced plasticity. We expect that plasticity changes after ischemia will be most pronounced in the cortex. Therefore we will examine areas of the sensorimotor cortex immediately adjacent to the region, or on the contralateral, homotopic cortex, which has also been demonstrated to be involved in plastic changes following ischemia. We will examine different time points after ischemia to observe the dynamics of changes, and gain additional information on the way regulated genes might be involved in plasticity processes. As ischemic model, we have chosen the photothrombotic model, which has the advantage of producing a small and defined cortical lesion Large infarcts as produced by the middle cerebral artery occlusion model (MCAO) are not ideally suited for this purpose, due to the fact that hemispheric infarctions result in severe neurological deficits and a subsequent high mortality. In addition to the genomic response evoked by different means of training, we will determine the contribution of voluntary versus forced training to functional neurological recovery. At present it is unclear how plasticity responses evoked by voluntary and forced training paradigms relate to each other, and whether one of those paradigms is superior to the other. For measuring the amount of recovery, groups of animals subjected to different training paradigms for 4 weeks after stroke will be monitored for performance in daily living activities, motor performance in rotarod, cylinder- and tape removal test, and neurological outcome score. Thus, we will be able to correlate the genomic profile obtained to behavioral performance of the rats.
The photothrombotic model
An elegant way to produce circumscribed cortical infarcts is the photothrombotic model (Markgraf et al., 1993; Wester et al., 1995; Wood et al., 1996, Schäbitz et al. 2004). Here, the dye rose bengal is administered to the animals intravenously, followed by illumination of part of the cortex with a light source. This produces small, consistent infarcts. This model is well suited for evaluating functional outcome with sophisticated tests (Wood et al., 1996; Rogers and Hunter, 1997), and search for genes involved in plasticity. A PE-50 polyethylene tube is inserted into the right femoral artery for continuous monitoring of mean arterial blood pressure, and blood gases. The right femoral vein is cannulated by a PE-50 tube for treatment infusion. During the experiment rectal temperature is monitored and maintained at 37 °C by a thermostatically controlled heating pad (Föhr Medical Intruments, Germany).
Photothrombotic ischemia is induced in the rat parietal cortex according to the method of Watson et al.. Animals are placed in a stereotaxic frame, and the scalp is incised for exposure of the skull surface. For illumination, the skull is illuminated with a Laser light source for 5 minutes. Immediately prior to the illumination, the dye rose bengal (0.133 ml/kg body weight, 10 mg/ml saline) is injected intravenously. Sham-operated animals undergo the same experimental procedures as described above without infusion of rose bengal and illumination. After surgery, the catheters are removed, and the animals are allowed to recover from the anesthesia and given food and water ad libitum.
Setup
“Voluntary” training of the animals will be stimulated by connecting a freely accessible running wheel to the cages of the animals equipped with a monitoring device. The “forced” training by forced arm use (FAU) will be modeled by applying a 1-sleeve plaster cast onto the ipsilateral limb.
Functional outcome measurements
Functional outcome will be measured using a battery of sensory-motor tests. The tape removal test, the cylinder test, rotarod, and a neurological outcome score (NSS). In all animals a battery of behavioral tests is performed at baseline before ischemia, after ischemia, after the training period of 10 days, and at 4 weeks after ischemia by an investigator blinded to the experimental groups. For the rotarod test, rats are placed on an accelerating rotarod cylinder, and the time the animals remain on the rotarod is measured. The speed is slowly increased from 4 to 40 rpm within 5 minutes. The trial ends if the animal fall off the rungs or grip the device and spin around for 2 consecutive revolutions without attempting to walk on the rungs. An arbitrary limit of time is set for the rats at 500 seconds on the rotarod cylinder in training as well as in testing procedures. The animals are trained 3 days before ischemia. The mean duration (in seconds) on the device is recorded with 3 rotarod measurements 1 day before surgery. Motor test data are presented as percentage of mean duration (3 trials) on the rotarod compared with the internal baseline control (before surgery). For the adhesive-removal test, the rats are at first familiarized with the testing environment. Two small pieces of adhesive-backed paper dots (of equal size, 113 mm²) are used as bilateral tactile stimuli occupying the distal-radial region on the wrist of each forelimb. The rat is then returned to its cage. The time to remove each tape from the forelimbs is recorded on 5 trials per day for each forepaw. Individual trials are separated by at least 5 minutes. Before surgery, the animals are trained for 3 days. Once the rats were able to remove the dots within 10 seconds, they were subjected to ischemia. The Neurological Severity Score (NSS) is used in a modified version. Neurological function is graded on a scale of 0 to 16 (normal score, 0; maximal deficit score, 16). NSS is a composite of motor, sensory, and reflex tests and includes the beam balance test. In the severity scores of injury, 1 score point is awarded for the inability to perform the test or for the lack of a tested reflex; thus, the higher score, the more severe is the injury.
RNA extraction and DNA-Array hybridization
Samples will be taken from the ipsi- and contralateral cortex using punch biopsies. RNA is initially prepared by a guanidinium-isothiocyanate acid-phenol extraction, after homogenizing the tissue with an Ultraturrax T25 (Ilka Labortechnik, Staufen, Germany). This step is followed by further purification using RNAeasy columns (Qiagen, Germany). RNA integrity and concentration is determined by photometric measurements (Agilent Bioanalyzer), and samples are stored at –80°C. The complete available rat Genome chip will be used (Affymetrix GeneChip® Rat Genome 230 2.0 Array), encompassing all known genes and ESTs for the rat. Affymetrix hybridisations will be done on a service basis (e.g. RZPD, Berlin), results will be analyzed using Affymetrix software and Genesifter (www.genesifter.com).
Project Status and Outlook
Establishment of the model
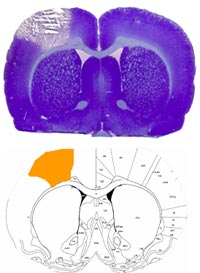
Previously, we have used the photothrombotic model with by injecting the dye rose bengal followed by illumination of the skull with a conventional white light source. We have now improved the model with a laser illumination (Krypton laser, 568 nm, 30mWatts) for a better reproducibility of a defined lesion. We determined the coordinates (-0.5 mm anterior, 3 mm lateral to the Bregma, laser diameter of 5 mm) as suitable to produce reliable infarcts in the right forepaw S1/M1 fields (see Figure 1) with a paresis of the left forearm. The surgical procedure itself including femoral catheter placement is established and takes 60 min.
After finishing a series of pilot studies with the modified photothrombotic model, we are currently applying both training paradigms (voluntary running, forced training), and assessing behavioral parameters. We plan to start with the final animal experiments end of 2005. After acquisition and dissection of the brains we will begin to conduct the array experiments in search for regulated genes in 2006.
Lit.: 1. Schabitz W. R., et al. (1999) Synergistic effects of a combination of low-dose basic fibroblast growth factor and citicoline after temporary experimental focal ischemia. Stroke 30, 427-431; discussion 431-422. 2. Schneider A., et al. (1999) NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med 5, 554-559. 3. Fisher M. and Schaebitz W. (2000) An overview of acute stroke therapy: past, present, and future. Arch Intern Med 160, 3196-3206. 4. Schabitz W. R., et al. (2000) Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke 31, 2212-2217. 5. Schabitz W. R., et al. (2001) Delayed neuroprotective effect of insulin-like growth factor-i after experimental transient focal cerebral ischemia monitored with mri. Stroke 32, 1226-1233. 6. Scheel J., et al. (2002) Yellow pages to the transcriptome. Pharmacogenomics 3, 791-807. 7. Sommer C., et al. (2003) Exogenous brain-derived neurotrophic factor prevents postischemic downregulation of [3H]muscimol binding to GABA(A) receptors in the cortical penumbra. Brain Res Mol Brain Res 111, 24-30. 8. Schneider A., et al. (2004) Restriction-mediated differential display (RMDD) identifies pip92 as a pro-apoptotic gene product induced during focal cerebral ischemia. J Cereb Blood Flow Metab 24, 224-236. 9. Schneider A., et al.. (2004) Identification of regulated genes during permanent focal cerebral ischaemia: characterization of the protein kinase 9b5/MARKL1/MARK4. J Neurochem 88, 1114-1126. 10. Schabitz W. R., et al. (2004) Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke 35, 992-997. 11. Schneider A., et al.. (2005) The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest 115, 2083-2098.


