
Introduction
Subtle changes in the amount of gene products or their transient modifications are the molecular cause for major health problems. Highly sensitive and specific detection methods are required for the systematic large-scale quantification of these alterations. We have developed a quantitative immunoblotting assay based on chemiluminescence in combination with CCD camera detection to quantitate specific proteins and their transient modifications. A separation according to the molecular weight is essential, since antibodies frequently detect unrelated proteins in complex mixtures like cellular lysates or serum. As an advancement of this method, antibody-based protein arrays have been developed to detect multiple proteins simultaneously in complex mixtures. This method is based on the direct or indirect detection of proteins using fluorescently labeled proteins. However, signal specificity and resolution are very limited. The use of nanocrystals (quantum dots) shall improve the output of the arrays. In contrast to traditional fluorophores, functional nanocrystals show wide absorption profiles, composition-tunable narrow emission and excellent photostability. To establish a highly sensitive and specific method for the quantitative detection by protein arrays we will immobilize capture antibodies on slides and use specific antibodies labeled with nanocrystals for detection. The application of near-infrared nanocrystals with different emission spectra will facilitate simultaneous detection of multiple proteins/modifications and help to greatly increase the sensitivity. To compensate for the lack of in gel separation and to ensure specificity, we will apply sets of two labeled antibodies recognizing neighboring epitopes on the protein of interest. The strength of specific signals will be quantitated by a special case of fluorescence resonance energy transfer (FRET) measuring fluorescence quenching between the labels attached to the detection antibodies. Due to the broad excitation spectra of nanocrystals the measurement of spacial approximation by conventional FRET is not possible, but can be quantified by energy transfer from nanocrystals to for example gold particles (quenchers) resulting in a detectable reduction of the emitted nanocrystal fluorescence in the near-infrared range (quenching) For quality control the results obtained will be compared to quantitative immunoblotting determinations. Statistical signal processing will be used to convert the raw data into calibrated estimates of protein abundance.
Project Status
Project Klingmüller:
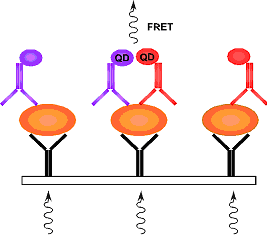
Fig 1: Specific detection in the protein array by nanocrystals (quantum dots) in the near-infrared range. The protein of interest (orange) is immobilized by a capturing antibody and detected by detection antibodies) coupled to nanocrystals (QD; purple) or quencher (QE; red).
Based on our extensive experience, we are using the suspension cell line BaF3 and adherent Hepa1-6 or primary hepatocytes to monitor the activation of STAT5, STAT3, ERK1, ERK2, SMAD2 and SMAD3. To ensure comparability of experiments and reduce the noise in the detection process reliable quantification of cellular lysates is important. Sophia Derdak has established procedures using the BCA protein assay kit that provide sufficient accuracy and resolution to allow a direct correlation between the protein concentration and the number of lyzed cells. These procedures are documented as standard operating procedures (SOP) and are shared with the project of Ulrike Korf.
Furthermore large batches of recombinant proteins are required as reference to permit comparable measurements in immunoblot and protein array through out the project. Sophia Derdak has successfully prepared large batches of recombinant SMAD2 and SMAD3 and is currently in the process of preparing recombinant STAT3. Recombinant ERK1 and ERK2 are currently being prepared by Ulrike Korf. Since chemiluminescence in combination with the LumiImager is currently being used for quantifiaction of immunoblots whereas for the protein arrays we are applying the Odyssey system from Li-Cor that is making use of fluorescent labeled antibodies operating in the near-infrared range. It is important to determine whether similar absolute values for protein concentrations can be obtained by both devices. Sophia Derdak is currently analyzing immunoblots in parallel by both detection systems and is comparing the results. To establish antibodies suitable as capture antibody and for the detection of separate epitopes she is screening together with Ulrike Korf commercially available polyclonal and monoclonal antibodies against ERK1 and ERK2, STAT3 and STAT5 and SMAD2 and 3. For the in silico identification of epitopes for antibody recognition she has employed together with Tim Beissbarth (project Huber) the SwissProt, pdb and other structural protein databases and is currently designing experimental tests to substantiate the findings. Furthermore, in collaboration with Frank Riehle (project Nann) she is in the process of preparing a modified Protein A construct for use as detection reagent coupled to nanocrystals.
Project Korf:
To compare the detection of proteins under denaturing or non-denaturing conditions in the protein array Ulrike Korf has in collaboration with Sophia Derdak (project Klingmüller) optimized the preparation of cellular lysates of BaF3-EpoR and BaF3-EpoRgp130 cells. The cellular lysates were initially used to screen antibodies for the detection of non-phosphorylated and phosphorylated forms of ERK1/2 and STAT3 by immunoblotting. Thereby she has identified antibodies that can specifically detect the protein of interest in immunoblots.
These antibodies serve as basis for the microarray-based indirect detection of ERK2 and STAT3. Highly specific mouse monoclonal antibodies were immobilized as capture antibodies. Different specific polyclonal antibodies were experimentally compared for their suitability as detection antibody. Routinely, subarrays are printed on a 16-pad slide. Thereby, individual subcompartments are generated that permit the analysis of multiple samples e.g. individual time points per microarray. The visualization is based on near infrared detection of fluorescent dyes coupled to anti-rabbit antibodies. Ulrike Korf could demonstrate in collaboration with Achim Tresch (project Huber), that the optimal sensitivity range of different monoclonal capture antibodies differs greatly. Currently, the detection of ERK2 is feasible in the range of 0.1 ng/ml up to 100 ng/ml. In addition, cytosolic ERK2 could specifically be detected in cellular lysates. The array-based calculation of the ERK2 abundance corresponds to the numbers determined in the laboratory of Ursula Klingmüller by immunoblotting.
Project Nann:
The preparation of luminescent II/VI-semiconducting nanocrystals (quantum dots) for biological applications is a complicated process containing four synthesis steps. (1) In the first step the core, that defines the opto-physical properties like fluoresence, is synthesised in a complex organic matrix. (2) To protect the nanocrystals from chemical degradation, the core has to be passivated by a ZnS-shell. (3) The next step, which is the most difficult, transfers the nanocrystals into the aqueous phase by chemisorption of single molecules forming a self-assembling monolayer (SAM). (4) The watersoluble nanocrystals are functionalized by specific organic linkers and are finally ready (biocompatible) to bind proteins. For the use of nano-bio-conjugates in quantitative protein arrays the nanocrystal-tagged proteins must be carefully characterized and purified. Particularly important to ensure a wide detection range of our protein arrays is that nanocrystals of high quality (high quantum yield, highly monodisperse) are available. Frank Riehle has improved as a II/VI-model system the synthesis of CdSe-nanocrystals (core-synthesis) and developped a simple reproducible method to obtain highly monodisperse and highly luminescent nanoparticles in large amounts which also works for the synthesis of in near infrared emitting CdTe-nanoparticles. Concepts for generating specific nano-bio-conjugates, their purification and characterisation are being established and experiments are in preparation.
Project Huber:
To facilitate data-management and ensure standardized documentation of experimental results as well as their bioinformatic processing Achim Tresch has set up in collaboration with Florian Hahne (DKFZ) an SVN Repository, "quantmod". This is platform is now being used by all project members for data storage, documentation and exchange. Furthermore, Achim Tresch has written algorithms for acquisition and processing of data generated by protein arrays in collaboration with Ulrike Korf. In addition he has employed and developed statistical modeling for the reliability assessment of the data and its proper pre-processing. To identify potential epitopes for antibody recognition Tim Beissbarth together with Sophia Derdak (project Klingmüller) has scanned the pdb database for protein structures of ERK1, ERK2, STAT3, STAT5, SMAD2 and SMAD3.
Outlook
The detection with nanocrystals in biological systems is an emerging field. We are combining the advantages of near infrared nanocrystals with adapting and miniaturizing classical antibody-based techniques to develop highly sensitive and specific quantitative protein arrays. This will facilitate the systematic large-scale quantification of proteins in complex mixtures, a technology that is in high demand for systems biology approaches and diagnostic purposes.


