
Introduction
Mouse mutagenesis is one of the most powerful approaches for the analysis of gene-specific functions. However, a critical limitation in the investigation of brain function in, for example, mutant mouse disease models is the lack of efficient, high-resolution in vivo analyses of neuronal networks. Up to now such studies have involved, at best, extensive in vitro (slice) electrophysiological recordings from individual cells, necessarily isolated from the brains network. The extend to which such in vitro studies reflect biologically relevant functions remains in most cases unclear. We propose the development of in vivo brain endoscopy for the analysis of neuronal networks with single cell resolution. These approaches involve fluorometric Ca2+ recordings through optical fibers and two-photon microscopy.
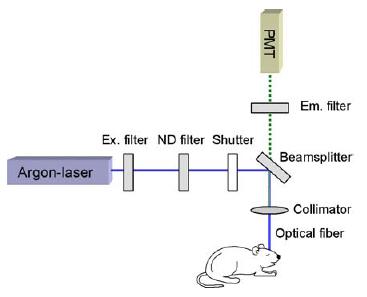
Fig 1: Scheme of the optical fiber based calcium imaging system.
Alterations of the intracellular Ca2+ homeostasis have been shown to play a central role in the pathogenesis of several age-related neurodegenerative diseases, like Alzheimer´s or Parkinson´s disease. The majority of many earlier studies, however, were performed in vitro in cultured embryonic cells. It is, therefore, difficult to relate those results to specific patho-mechanisms of the disease, because at early developmental stages mutant animals often lack disease-related phenotypical changes. Thus, the analysis of normal brain function as well as that of the underlying pathophysiological mechanisms of neurodegenerative diseases requires in vivo methods for detection of neuronal calcium signaling in adult and aged brains.
To monitor changes in the intracellular calcium concentration, cells are usually stained with Ca2+-sensitive fluorescent indicator dyes. Upon binding Ca2+ ions such dyes change the spectral characteristics of the emitted light. The emitted light is collected either by CCD- (charge-coupled device) or video-camera-based systems or by photomultipliers. In vivo Ca2+ measurements require photomultiplier-based two-photon imaging because it is the most sensitive approach for measuring fluorescence in scattering opaque tissues. So far, however, such measurements have been restricted to anaesthetized or fixed animals and have been outermost performed only in the upper cortical layers. We propose the implementation of endoscope-like devices for Ca2+ measurements to gain access to any brain region of interest. That this is feasible has is indicated by recent work describing the use of a pair of implanted optical fibers, one for the excitation of a Ca2+-sensitive dye, the other one for detection of the emitted fluorescence [1].
Project Status
In a previous study we found wave-like cortical Ca2+ activity in brain slices and whole brain explants of newborn rats and mice, termed cortical early network oscillation (cENOs) [2]. They occur spontaneously and are caused by synchronous activation of a large number of neurons. In vitro such Ca2+ oscillations were shown to control neuronal maturation, synaptic wiring and regulation of neurite growth as well as regulation of gene expression in the developing brain. However, demonstration of their existence in vivo, in the intact brain subjected to a variety of sensory stimuli remained elusive. To conduct such in vivo experiments we have developed a technique for bulk loading of cells with membrane-permeable Ca2+ indicator dyes [3]. Two-photon Ca2+ imaging has shown that stained cells are viable and responding to sensory stimulation such as, for example whisker deflection. However, no spontaneous cENOs are observed in the cortex of neonatal mice suggesting that they might be blocked by the anesthetics. To monitor Ca2+ levels in awake behaving mice we have developed a prototype of an optical fiber-based recording system consisting of a chronically implanted fiber with 200 µm diameter used both for excitation of the dye and for detection of the emitted fluorescence (Fig. 1). This “single-fiber endoscope” allows recording of Ca2+ signals from any brain region in anesthetized as well as awake, behaving animals. It offers therefore a unique opportunity for studying brain function and for detecting the action of drugs without the influence of anesthetics. In addition it is also applicable to cultured cells and can therefore be enhanced to an automated high throughput screening (HTS) platform for testing of drug candidates influencing Ca2+ channel function.
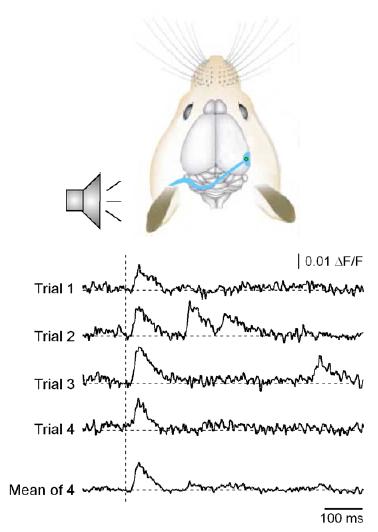
Fig 2: Sound-evoked calcium transients detected with the optical fiber in the primary auditory cortex of an adult mouse.
For detecting light-evoked transients the optic fiber was implanted in the right primary visual cortex. Stimulation was performed with a white LED. To test the specificity of the stimulation the light flashes (100 ms length, 10 s stimulus interval) were applied either through the ipsilateral or through the contralateral eye. Only in the latter configuration stimulus-evoked calcium transients were detected. These appeared with a mean delay of 68.6 ms after onset of the stimulus. The delays of the calcium transients detected after stimulation of the ipsilateral eye varied randomly, suggesting that in this case only spontaneous transients are present. Stimulus-evoked transients could also be detected with the optical fiber in the hippocampus and in secondary cortical regions, like the entorhinal and the frontal cortex. They appeared with delays longer than the ones in the respective primary sensory regions. Open questions are whether spontaneous and stimulus-evoked transients are generated by the same group of neurons or by distinct populations and the temporal relation between primary and secondary sensory regions and deeper brain regions like the hippocampus and the thalamus. Furthermore the influence of the anaesthesia on the physiological brain signalling has to be investigated by performing recordings in awake animals.
Outlook
Our future work will focus on the development of the following approaches for the functional analysis of physiological and pathological brain function in vivo:
1. Establishment of a “double-fiber endoscope” combined with a video tracking system for the simultaneous analysis of calcium signaling in different brain regions and behavior in non-anesthetized mice.
2. Development of GRIN lens based micro-endoscopic devices for two-photon imaging and their implementation for in vivo applications. Such devices allow imaging with a high temporal and spatial (=cellular) resolution from any brain region of interest.
3. Alterations in brain calcium signaling will be tested in mutant mouse models of Alzheimer´s disease.
Lit.: 1. Duff Davis M., Schmidt J.J. (2000) In vivo spectrometric calcium flux recordings of intrinsic caudate-putamen cells and transplanted IMR-32 neuroblastoma cells using miniature fiber optodes in anesthetized and awake rats and monkeys. J. Neurosci. Meth. 99:9-23. 2. Garaschuk O., Linn J., Eilers J.& Konnerth A. (2000) Large-scale oscillatory calcium waves in the immature cortex. Nat. Neurosci., 3, 452-459. 3. Stosiek C., Garaschuk O., Holthoff K & Konnerth A. (2003) In vivo two-photon calcium imaging of neuronal networks. PNAS USA, 100, 7319-7324. 4. Adelsberger H., Garaschuk O., Konnerth A. (2005) Cortical calcium waves in resting newborn mice. Nature Neurosci., Jul 10; [Epub ahead of print]


