
Introduction
Much of the pioneering work in the genetics and biology of longevity has been elucidated in the nematode C. elegans. Several molecular pathways have been identified that extend life span in nematodes, flies and mice. Despite the progress in these model organisms, a large-scale, systematic approach for the exploration of the genetic basis of longevity in humans has not yet been undertaken. The main focus of this explorative project (EP) is to map and characterize genetic susceptibility factors that predispose to healthy longevity in humans as well as to identify molecular pathways that are associated with the physiology of ageing and/or age-related disorders and diseases.
This EP is a multi-center study to which the following institutes contribute: Institute for Clinical Molecular Biology, University Hospital Schleswig-Holstein, Kiel (Prof. Stefan Schreiber); Department of Dermatology, Charité Universitätsmedizin Berlin (Prof. Christos C. Zouboulis); Max Planck Institute for Molecular Genetics Berlin (Prof. Hans Lehrach). The members of this EP cooperation are using the existing NGFN structures involving various SMPs (e.g. SMP-DNA, SMP-RNA and SMP-Bioinformatics) at different locations to create synergisms in many aspects. The overall goal of the EP is achieved by employing an integrated and interdisciplinary approach, which applies cutting-edge technologies from different disciplines. The methodologies are state-of-the-art, comprising human positional cloning, human (skin cell cultures and biopsies) and animal models (C. elegans and M. musculus) and functional genomics (Fig. 1).
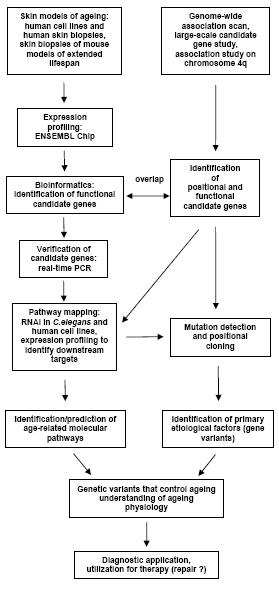
Fig 1: Exploration strategy of the project “Genetic Etiology of Human Longevity“
Those positional and functional candidate genes validated through expression and RNAi experiments are further characterized in humans by application of comprehensive mutation detection and high-throughput genotyping. This EP offers therefore a unique chance to identify the genetic susceptibility components for human longevity. The model systems included in this EP will be of seminal importance for the functional evaluation of the identified genetic variants that are relevant to the longevity phenotype.
Project Status
For the detection of genetic variants influencing the longevity phenotype in humans, association mapping is currently being carried out at the Institute for Clinical Molecular Biology in Kiel. More than 2200 individuals aged 90 years and older have so far been recruited (436 of these are centenarians). A large-scale candidate gene study is in progress: approximately 1300 coding SNPs are analyzed in about 880 functional candidates from pathways shown in model organisms to be involved in longevity and ageing (e.g. DNA metabolism, anti-oxidation, apoptosis). The typing is performed in 1104 DNA samples each of long-lived individuals (= 95 years) and younger controls (60-75 years) using the novel SNPlex high-throughput technology (Applied Biosystems) recently established at the Institute in Kiel. In addition, studies to examine the role of genetic variation in a number of functional genes have already been conducted. In contrast to a previously published report2, we have shown that a noteworthy influence of gene variation in the microsomal transfer protein (MTP, located in the candidate region on chromosome 4q1) on human longevity can be ruled out in the German samples analyzed3. Complementary to that approach, model systems for human ageing are screened with DNA microarrays. The Charité Department of Dermatology and the Max Planck Institute for Molecular Genetics in Berlin have conducted a high throughput study involving approximately 15,000 different human genes with reproducible in vitro models of hormonally induced human ageing. 899 genes showed altered expression due to the ageing process. Using bioinformatics methods these genes were associated with their corresponding metabolic and signalling pathways. Selected markers have been validated by RT-PCR and are currently screened in C.elegans for their effect on longevity.
Outlook
This EP relates to a pressing social problem. The German society is greying rapidly as evidenced by a growing percentage of individuals in the old-age groups. The proportion of the over-60 year-old individuals is estimated to rise from approximately one quarter in 2001 to more than one third in 2050. The proportion of 80-year-olds and older will almost triple, and could reach 12% in 20504. The consequences of this demographic trend, which is also due to a low birth rate, are challenging public policies in decades to come. To lessen the resulting social, economic and health burdens, it is crucial that the biological and non-biological causes for longevity be fully elucidated. While the discovery of gene variants and molecular pathways associated with healthy longevity is our primary interest, the project may also yield insights into the genetic causes of population disorders. This EP is expected to yield fundamental knowledge that may facilitate the development of regenerative drug strategies for the treatment of age-related and/or degenerative diseases. In addition, the establishment of new molecular diagnostic tools and the invention of patentable technology are industry-relevant targets and relevant for commercial exploitation. One possible outcome of the project is an improved understanding of repair mechanisms.
Lit.: 1. Puca AA et al. A genome-wide scan for linkage to human exceptional longevity identifies a locus on chromosome 4. Proc Natl Acad Sci USA 2001; 98: 10505-10508. 2. Geesaman BJ et al. Haplotype-based identification of a microsomal transfer protein marker associated with the human lifespan. Proc Natl Acad Sci USA 2003; 100: 14115-14120. 3. Nebel A et al. No association between microsomal transfer protein (MTP) haplotype and longevity in humans. Proc Natl Acad Sci USA 2005; 102: 7906-7909. 4. Federal Statistical Office of Germany, 2003 (http://www.destatis.de).


