
Introduction
Scanning microprobe matrix-assisted laser desorption / ionization mass spectrometry (SMALDI-MS) with infrared (IR) emitting desorption lasers is an instrumentally and methodologically new approach with an enormous potential for tissue analysis. Its capability of analyzing and visualizing single molecule distributions in biological tissue will have great impact on e.g proteomics and metabolomics research. Correlation of histological phenomena with molecular images will provide for a new quality of medical diagnostics and molecular understanding of diseases.
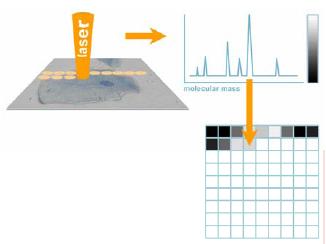
Fig 1: Scanning microprobe MALDI mass spectrometry: scheme of the process. A pulsed laser scans the surface (e.g. a biological cell), producing a mass spectrum for each spot. The intensity of a selected mass signal (a molecular species or “biomarker”) is transformed into a greyscale value and is drawn to the according pixel map.
Besides the less aggressive fragmentation behavior, IR-MALDI presents larger penetration depths, allowing the practical detection sensitivity of trace components to increase. Focusing an infrared laser radiation to the size of micrometers (microprobe mode), however, is much more demanding than focusing of ultraviolet laser radiation, due to physical laws of wavelength-restricted focal diameters. The goal of the project is to get close to a focus diameter of 5 µm using a laser wavelength of 2.8 µm to 2.9 µm. Another technical problem of scanning microprobe MALDI-MS is the general necessity of covering or mixing the analyte with a matrix substance. Most MALDI-compatible matrices are mixed with the analyte in solution and are subsequently dried on target or stay liquid when introduced into the high-vacuum chamber of the MALDI mass spectrometer. This process does not cause any problem for conventional MALDI, but it potentially changes the spatial distribution of certain analyte molecules in the tissue to be scanned. The migration and consecutive destruction of lateral information can be avoided by appropriate preparation protocols.
Such methods have been developed for UV matrices but do not yet exist for IR matrices. To make things more difficult, there are not as many well-working matrices known for IR-MALDI as they are known for UV-MALDI. On the good side, however, IR-MALDI is known to have the potential of analyzing bio-molecules without externally adding any matrix substance, by just using the residual water of the sample as a matrix [4].
Finally, to evaluate the new IR-SMALDI device, well-defined standards of structured analyte systems and tissues have to be developed and characterized by employing other established methods. IR-SMALDI-MS will be of high commercial interest to manufacturers of mass spectrometers, since imaging mass spectrometry in general is gaining considerable importance worldwide [5]. The project is highly explorative, since the technological and methodological goals are novel and risky but very promising. To ensure the objective and applicative integration in the road map of the NGFN, a direct cooperation exists with the clinical project (KG) “Infection and Inflammation” (Prof. Chakraborty). Further collaborations within the open structure of the NGFN are planed.
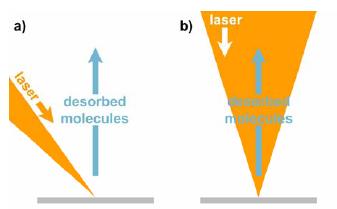
Fig 2: Laser desorption and ion beam geometry: a) In conventional MALDI mass spectrometers, the desorption laser hits the target surface at about 45°, projecting an ellipse. b) A coaxial laser beam allows minimum focus diameters with circular profiles.
The initial phase of the project is dominated by the planning and step-by-step construction of the new instrumental setup. As expected, one of the biggest challenges is to find an appropriate pulsed IR laser system. Er:YAG solid state lasers tend to be unstable in radiation power and performance and are not offered by any manufacturer worldwide at this time. Optical parametric oscillators (OPO) are wavelength-tunable but very cost intensive and they involve intense servicing and maintenance.
Right now, we are experimenting with highly promising, robust, and comparably inexpensive alternatives based on Raman conversion. There is no commercial experience yet in this field for the desired wavelength range, and experiments are underway in our lab in cooperation with manufacturers. As soon as the laser system is specified, the special laser optics will be designed and ordered. This focusing lens system is a dedicated construction and will allow for the desired small laser focus. In the meantime the surrounding analytical system will be set up to allow instant research work as soon as the complete laser system is operable.
Experiments will be carried out on two different types of mass spectrometers: a home-built linear time-of-flight mass spectrometer (TOF-MS), and a commercially available linear trap / Fourier-Transform Ion Cyclotron Resonance MS (Thermo Finnigan LTQ FT). Both devices require modification for the IR-SMALDI project. The TOF-MS is controlled by an outdated, MS-DOS based software package with severe hardware limitations. The LTQ FT does have an x-y stage, but this does not include a z direction as is needed for careful target adjustment and surface scan. A completely new ion source is needed for both devices. In addition some other units auch as IR laser beam attenuators have to be implemented.
To keep the computer programming work low and at the same time to introduce a flexible interface on a long-term view, a USB controller board was constructed. The driver is open source to be adjustable to future operating systems. Both mass spectrometers will be altered such that IR mode can be switched to UV mode and vice versa with only little work. In order to reduce lateral migration of analytes in tissue during preparation, matrices have to be investigated and tested in detail and protocols have to be developed. Today, there are only two IR matrices known which provide reproducible mass spectra of good quality: succinic acid (solid state) and glycerol (liquid state). Both substances were ordered from several different commercial providers, since it is known that the technical quality and purity of chemicals can have a strong effect on analytical results in MALDI-MS. They will be systematically compared and optimized. One of the major topics is contamination with sodium, potassium, and residual buffers, all of them representing factors to decrease the quality of spectra and analytical sensitivity, especially with nucleic acids as analytes. Different purification methods will be tested. The imaging software that is necessary for producing images from mass spectrometric data (developed by our group) was expanded to the needs of the IR-SMALDI project. The wider mass range of IR-SMALDI compared to UV-SMALDI, for example, required a modification of the auto-labeling algorithm, which now has to take into account a non-linear decrease of mass resolving power with increasing molecular mass within a mass spectrum. Cooperation with partners for the preparation of appropriate tissue thin sections was started.
Outlook Bild rechts:
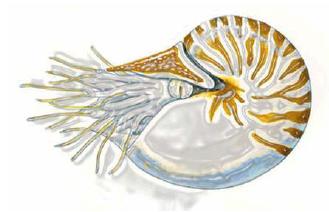
Fig 3: Nautilus pompilius (cephalopod): preliminary model organism for the project. Certain compartments contain localized pheromones and may serve as a standard to define e.g. the scanning resolution and reproducibility of the new IR-SMALDI-MS.
The project started well off, encountering the expected difficulties in finding a laser that meets all projected requirements. Until the new laser system is built and delivered, an old laser will be used to carry out preliminary experiments. The final setup will contain a dedicated lens system that will need about 10 months for construction and delivery. In the meantime we will use a conventional beam arrangement: in about all commercially available MALDI mass spectrometers, the laser hits the target at an angle of about 45 degrees. This geometry does not allow extremely small foci (which does not matter in non-scanning MALDI), but still we can collect a lot of experience and data that will accelerate future work with the dedicated laser optics.
For example, preparation and purification protocols can be checked and optimized, while the lateral spatial resolution of the surface scan will be reduced to the achievable minimum at a later stage of development.
Scanning microprobe MALDI with UV lasers was shown earlier to be a successful method in combination with a TOF-MS [6]. Transferring the method to a linear ion trap is difficult, since the ion optics is less tolerant. As a result of another research project, we lately succeeded with UV-SMALDI. Experiences from this project can now be used within the running IR-SMALDI project. For that, ion transmission has to be increased by adjusting the ion optics before IR-SMALDI can be introduced to the LTQ FT-MS.
To systematically compare and improve the IR-SMALDI method, it will be necessary to have access to well-defined biological tissue standards. The distribution of a certain protein must be reproducibly restricted to a tissue section while the protein shows high signal abundance in MALDI mass spectra. A cooperation with Dr. Westermann at Justus Liebig University Giessen might deliver such standard: The cephalopod Nautilus pompilius possesses a proboscis-like organ that is supposed to contain high amounts of pheromones. The organ is several centimeters in length and grants easy access to high concentrations of defined substances. First spectra from MALDI-TOF-MS and ESI-FT-MS show the dominance of a certain protein which we hope to identify within the next time.
Lit.: Spengler B., Hubert M.; J. Am. Soc. Mass Spectrom. 13 (2002) 735. 2. Dreisewerd K., Berkenkamp S., Leisner A., Rohlfing A., Menzel C.; Int. J. Mass. Spectrom. 226 (2003) 189 3. Berkenkamp S., Kirpekar F., Hillenkamp F.; Science 281 (1998). 4. Berkenkamp S., Karas M., Hillenkamp F.; Proc. Nat. Acad. Scienc. 93 (1996) 7003. 5. Hutchens TW.; Rapid Comm. Mass Spectrom. 7 (1993) 576. 6. Bouschen W.; Dissertation Univ. Giessen (2004).


