
Introduction
Serotonin (5-hydroxytryptamine, 5-HT) controls a variety of physiological functions in the central and peripheral nervous system. Most of the serotonin can be found in the gastrointesinal tract (95%) besides its distribution in brain and blood cells (Gershon, 2003). Serotonin action is mediated by a multitude of 5-HT receptor subtypes. These subtypes can be divided into seven main classes (5-HT1R to 5-HT7R) based on their structural and functional features (Hoyer et al., 2002). Except for the 5-HT3 receptor, which is a ligand-gated ion channel, all serotonin receptors represent G-protein coupled binding proteins. The ion channel itself is an oligomeric complex composed of five subunits. During the last years two different human 5-HT3 receptor subunit genes, HTR3A and HTR3B, have been isolated (Miyake et al., 1995; Belelli et al., 1995; Davies et al., 1999).
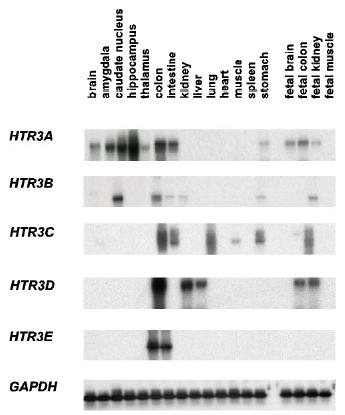
Fig 1: Expression analysis of HTR3 genes (Niesler et al., 2003).
Activation of the 5-HT3 receptor is involved in a variety of physiological effects in the central and peripheral neurons and has been implicated in a number of human diseases including anxiety, depression, anorexia, bulimia and irritable bowel syndrome (Gyermek, 1995; Graeff, 1997). The 5-HT3 receptor is involved in the control of gastrointestinal function and the discovery that 5-HT3 receptor antagonists inhibit chemotherapy and radiation induced emesis has revolutionised cancer therapy (Jones and Blackburn, 2002). Additionally, 5-HT3 receptor antagonists (e.g. alosetron, cilansetron, ondansetron) are successfully used in the therapy of irritable bowel syndrome (IBS) as well as eating disorders like anorexia nervosa (AN) and bulimia nervosa (BN) (Talley ,2001; Gershon, 2003). This makes the HTR3 genes to promising candidate genes in the etiology of gastrointestinal disorders.
Since HTR3E is the first serotonin receptor gene that is restrictedly expressed in the gastrointestinal system, this receptor subunit may be a major modulator of gastrointestinal function. With an incidence of approximately 10 % in the general population gastrointestinal disorders are frequent. Irritable bowel syndrome is associated with abdominal pain and abnormal bowel activity and may reflect hypersensitivity of the gastrointestinal tract to normal stimuli. The molecular basis of IBS as well as eating disorders is poorly understood, but there is evidence that serotonin plays a crucial role since it is a major neurotransmitter in the gastrointestinal tract. All mentioned patient groups show disturbances in the serotonin pathway. Interestingly, patients suffering from GI disorders frequently show comorbidity of psychiatric diseases, such as anxiety and depression. The ubiquitously expression of HTR3 genes combined with the restricted GI expression of HTR3E increases a possible potentiation of specific effects due to the variable molecular make-up of different receptor subtypes. Furthermore, ENS and CNS are directly connected via the nervus vagus and their crosstalking could influence mood or other brain functions directly. The bilateral nature of the relationship between ENS and CNS may explain the high frequency with which various psychoneuroses are correlated with functional and other diseases of the bowel (Gershon, 2003). These facts inspired us to the hypothesis that a variety of mutations within the HTR3 gene family may contribute to different types of gastrointestinal and associated neuropsychiatric disorders suggesting a broad range in phenotypic heterogeneity: Action of mutated gene products in concert may lead to a specific disease phenotype.
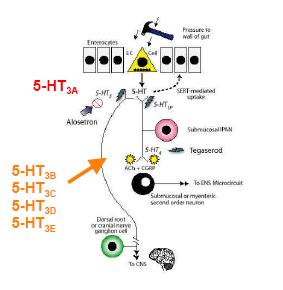
Fig 2: Serotonin in the eneteric nervous system (adapted fom Gershon, 2003).
The project is subdivided into two main parts: (A) Mutational analysis of HTR3 genes including the set up of the first serotonin receptor gene-specific data base and (B) functional characterisation of the 5-HT3 receptor system. In first instance, the putative role of HTR3 genes in the etiology of gastrointesintal disorders is investigated. The clarification of the pathomechanism of the disease will be achieved by screening patients and healthy controls by DHPLC analysis (denaturing high performance liquid chromatography). 300 blood samples of IBS patients as well as control persons are currently collected and consecutively analyzed. We will include patients suffering from Bulimia nervosa as well since bulimic patiens have efficiently been treated by ondansetron, a highly selective 5-HT3 receptor antagonist (Faris et al.,2000). Furthermore, Anorexia nervosa samples are part of the analysis. Genotype-phenotype correlations will give an insight into the consequences of certain mutations on phenotypic level. This picture might be completed by investigating gene-based haplotypes taking specific phenotypic features into account (associated psychiatric diseases, treatment using specific medication). In summary, the outcome of this initial screen represents a solid basis for further analysis in a pharmacogenetic approach investigating success of treatment of certain diseases using 5-HT3 receptor agonists/antagonists.
A large amount of data from a multitude of candidate gene screenings has been published during the last years. We will start to structure this information in the first 5-HT specific mutational database collecting SNPs (single nucleotide polymorphisms): This central information pool should help clinicians as well as scientists to evaluate detected mutations and to use the respective information for subsequent genotype-phenotype correlation studies and in pharmacogenetic approaches.
The second part of the project deals with the functional analysis and characterisation of the different HTR3 gene products. Preliminary data showed that the HTR3E protein alone is not able to form functional homopentameric 5-HT3 receptors (non-published data). Former data expressing HTR3B alone reflected the same situation: no functional homomeric receptors were detectable, whereas co-expression of HTR3A plus HTR3B revealed functional receptors showing distinct properties compared to homomeric 5-HT3A receptors (Davies at al., 1999). Co-expression of HTR3E with HTR3A should lead to functional ion channels. This data suppports the idea of 5-HT3E as a major modulator of 5-HT3 receptor function in the GI tract.
Receptor subtypes made up of different subunit compositions may exhibit different sensitivities to 5-HT3 receptor ligands, agonists as well as antagonists, and should consequently mediate specific responses to the drugs. The final goal is to enable the identification of highly selective drugs for improved therapy to avoid severe side-effects of nowadays used medication.
In parallel, we follow up investigating the precise role of the different subunits by biochemical and cytological investigations of recombinantly expressed proteins. Of course, the results achieved by analysis of the recombinant protein have yet to be validated by in vivo analysis of cells expressing the native protein endogenously. For this purpose subunit-specific antibodies are currently created as useful tools in the analysis of cytological and histological material. Studies in the past focussed mainly onto the mapping of 5-HT3 receptor distribution within the central nervous system. Consequently, the knowledge about presence within the enteric nervous system is poor. Hence 5-HT3 receptor mapping of the novel subunits may complete the puzzling picture how serotonin receptors are involved in control mechanisms of the gastrointestinal tract.
Outlook
The planned project may help to elucidate functional and regulatory processes in the highly complex organised enteric nervous system, in particular the ENS-CNS connection and the serotonin mediated neurotransmission. The identification of serotonin receptor gene variants, following genotype-phenotype correlations as well as pharmacogenetic studies may lead to the development of a possible diagnostic tool to predict appropriate treatment of the disease. Moreover, the outcome of the project may contribute to the identification and design of novel drugs for treatment of bowel diseases and concomitantly appearing psychiatric diseases. Furthermore, the HTR3 receptor gene database will represent a central source of information for clinicians as well as scientists interested in serotonin research and serotonin-related disorders. The project could also supplement the data of groups organised in the NGFN disease oriented network ‘Diseases of the nervous sytem’.
Lit.: 1. Belelli D et al. Mol Pharmacol Cloning and functional expression of a human 5-hydroxytryptamine type 3AS receptor subunit. 1995 48: 1054-62. 2. Davies PA et al. Nature The 5-HT3B subunit is a major determinant of serotonin-receptor function.1999 397: 359-363. 3. Faris PL et al. Effect of decreasing afferent vagal activity with ondansetron on symptoms of bulimia nervosa: a randomised, double-blind trial. 2000. Lancet 355:769-70. 4. Fletcher S and Barnes NM TiPS Desperately seeking subunits: are native 5-HT3 receptors really homomeric complexes?1998. 19: 212-215. 5. Gershon MD Rev Gastroenterol Disord Serotonin and its implication for the management of irritable bowel syndrome.2003 3: 25-34. 6. Graeff FG. Serotonergic systems. Psychiatr Clin North Am 1997 20: 723-739. 7. Gyermek L J Clin Pharmacol 5-HT3 receptors: pharmacologic and therapeutic aspects. 1995. 35: 845-855. 8. Hoyer D et al. Pharmacol Biochem Behav Molecular, pharmacological and functional diversity of 5-HT receptors. 2002. 71: 533-54. 9. Humphrey PPA et al. Aliment Pharmacol Ther Review article: the therapeutic potential of 5-HT3 receptor antagonists in the treatment of irritable bowel syndrome. 1999. 13, 31-38. 10. Jones BJ and Blackburn TP. The medical benefit of 5-HT research. Pharmacol Biochem Behav 2002. 71: 555-568. 11. Karnovsky AM et al. A cluster of novel serotonin receptor 3-like genes on human chromosome 3. Gene 2003 319: 137-148. 12. Miyake A et al. Molecular cloning of human 5-hydroxytryptamine3 receptor: heterogeneity in distribution and function among species. Mol Pharmacol 1995. 48: 407-416. 13. Niesler B. et al. Cloning, physical mapping and expression analysis of the human 5-HT3 serotonin receptor-like genes HTR3C, HTR3D and HTR3E. Gene. 2003 310:101-11. 14. Talley NJ. Serotoninergic neuroenteric modulators. Lancet 2001358: 2061-68.5-


