Introduction
Myocardial infarction (MI) and coronary artery disease (CAD) are leading causes of death in the western world. Besides known risk factors and their genetic background, a positive family history is an independent predictor, suggesting additional susceptibility genes. We intend to identify these genes using a family-based strategy and a genome-wide screening in a large set of MI families. Furthermore, within chromosomal regions that are linked to MI, we intend to identify those genes that are relevant for the development of MI.
The specific goals of our project are (1) to perform a linkage analysis in a second genome-wide screen in MI families; (2) to localise genes within regions with positive linkage signals from this second MI family set; (3) to perform linkage analysis for well defined intermediate risk factor phenotypes in all families (MI family sets 1 and 2); and (4) to analyse the functional relevance of genes and variants using different expression systems.
Results/Project Status
Phenotyping
Previously, we screened more than 200,000 MI patient charts in 17 cardiac rehabilitation clinics in Germany and established a respective DNA and database. We identified and extensively phenotyped 1,413 MI families (index patient suffering from MI at 60 years or younger (mean age 49.5 years) and at least one affected sibling suffered from MI, bypass surgery, or percutaneous coronary intervention at 70 years or younger (mean age 54.8 years). All participants were assessed by an extensive anthropometrical, clinical, and extensive biochemical protocol, including HDL and LDL cholesterol, triglycerides, Lp(a), HbA1c, and CRP levels.
In order to refine the cardiac phenotype, we have read all available coronary angiograms of all affected individuals. We retrospectively studied the coronary angiograms of 882 siblings with CAD from 401 families. Heritability calculations were performed with variance-component analysis. Traditional cardiovascular risk factors and age at the first coronary event displayed significant heritable components. After adjustment for age and sex, significant heritabilities were identified for proximal stenoses, in particular, left main CAD (h2=0.49±0.12; P=0.01), coronary calcification (h2=0.51± 0.17; P=0.001), and ectatic coronary lesions (h2=0.52±0.07; P=0.001). In contrast, no heritability was found for distal disease (h2=0.05±0.19; NS), the pattern of coronary arterial blood supply, or the number of diseased vessels. Calculation of recurrence risks in siblings largely confirmed the heritability estimates 1 .
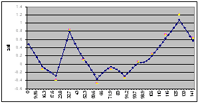
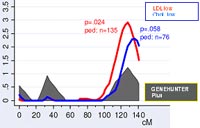
Linkage analysis
In previous work, we identified a susceptibility locus for CAD and MI on chromosome 14 using genome-wide linkage analyses in a subset of 513 families, corresponding to a total of 1,406 individuals 2. For the identification of chromosomal regions that may be relevant for MI or for cardiovascular risk factors, we have successfully performed a linkage analysis in a genome-wide screen in our second MI family set using several linkage programs (LINKAGE, GENEHUNTER) as well as the variance component analysis (SOLAR program). Within these evaluations, a peak could be seen in the area of chromosome 14 (Fig.1). When performing an analysis (ordered subset analysis), we identified a clear replication of the MI locus on chromosome 14 in subsets of MI families (Fig. 2).
The combined analysis of the full data sets of all families (MI family set 1 and 2) are underway and are expected to be finished until the end of 2005.
SNP identification and data mining
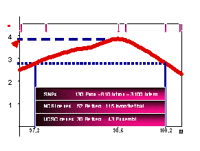
To identify genes within this region with positive and replicable linkage signal, we proposed to perform an association study using single nucleotide polymorphisms in the 95%-confidence area of our chromosomal region (fig. 3).
By genotyping a large number of SNPs in affected MI patients as well as in healthy control subjects, we intend to determine the genomic structure (incl. genes, intron-exon boundaries, SNPs, other polymorphisms) of areas identified by linkage analyses (here chromosome 14q32). Therefore, all information that is available in public databases was extracted and more than 7.000 SNPs were identified in our region, approximately 4000 of them were polymorphic.
SNP genotyping
In order to ensure the best cost-effectiveness, large-scale SNP genotyping was (and is going to be) performed in high throughput centres, such as SMP-DNA. In our ongoing project, a close collaboration with the GSF-Gene Mapping Centre has been successfully established.
In cooperation with the SMP-DNA at the GSF Munich, we planned to genotype 1.536 SNPs in the chromosome 14 area in 1.000 MI patients and 1.500 control subjects. When analysing the costs for this project, we decided in favour for the ILLUMINA platform. This technology has been made available in the SMP-DNA in Munich at the Institute of Prof. Meitinger. The ILLUMINA technology is very much depending on the quality and concentration of DNA, wherefore we have spend a more than three-month effort in preparing the samples. Furthermore, we have applied in the SMP-EPI at the GSF-Institute for epidemiology (Prof. Wichmann) for the use of DNA of healthy control subjects (KORA 2000). After the final approval of this application, we have normalized in our laboratory these samples as well as n=500 healthy control subjects from our Regensburg MI study population (married-in spouses).
DNA from all individuals was then transferred to the laboratory of Prof. Meitinger (SMP DNA, GSF Munich). The chemical reactions necessary for detection of the genotype signal were performed in this laboratory.
A total of 1.536 SNPs were selected and the order for prepareing the arrays at ILLUMINA in San Diego (USA) was placed after our detailed cost planning was agreed by the DLR.
At present, the genotyping is in the last phase and we expect the last DNA to be genotyped by the end of August 2005.
In September 2005, it is planned to evaluate the first genotyping results and to perform descriptive statistics (such as allele frequency testing, Hardy-Weinberg testing, heterogeneity testing) as well as more detailed haplotype analysis. A linkage disequilibrium map will be established in order to better characterise our region and to facilitate further case-control studies for confirmation of the possible positive SNPs.
Genomic work and sequencing
Furthermore, sequencing in specifically interesting areas with insufficient SNP coverage to identify new gene polymorphisms will complete the genomic work of the region. Moreover, it is intended to sequence specific candidate genes in identified genomic regions in 24 individuals.
Association studies
Newly identified SNPs and the most informative SNPs (total 250 SNPs) will be evaluated in association studies of 700 MI cases and 700 controls with (total genotypes 350.000). Results of SNP genotyping and SNP haplotype analysis will be validated in independent unselected MI patients (n=4.000) and population-based control populations (n=4.000): 30 SNPs in MI cases and controls from Regensburg, Lübeck, Augsburg, München, and Aachen (total genotypes 240.000). It is to be expected that these large numbers will be necessary to give sufficient power to detect significant effects of a single gene in an unselected population in which environmental and other genetic effects will dilute the influence of a tested polymorphism. Finally, candidate genes in genomic regions linked to risk factors in the MI families will be analysed by association studies in the general population (total genotypes 80.000).
Functional studies
Since no specific gene targets have been identified, functional studies have not yet begun.
Outlook
The overall goal of this subproject is to identify genetic risk factors for myocardial infarction (MI) and atherosclerosis. For this purpose, a detailed evaluation of a MI locus on chromosome 14q32 is being performed using linkage studies, candidate gene analysis, case-control association studies, and - in the future - functional studies. The identification of risk loci for MI will prove the translation of genetic examinations to functional studies in model organisms and subsequently into clinical practice.
Lit.: 1. Fischer M, Broeckel U, Holmer S, Baessler A, Hengstenberg C, Mayer B, Erdmann J, Klein G, Riegger G, Jacob HJ, Schunkert H. Distinct Heritable Patterns of Angiographic Coronary Artery Disease in Families With Myocardial Infarction. Circulation. 2005;111:855-862. 2. Hengstenberg C, Broeckel U et al. A comprehensive linkage analysis for myo¬cardial infarction and its related risk factors.Nat Genet. 2002;30(17)210-4:.


