Introduction
In human, blindness is caused by several different ocular diseases. Among these, the cataracts are responsible for half of all cases (1). The retinal disorders cover a broad variety of clinical symptoms and many different genes are involved in the corresponding pathological conditions in human. The two most important groups are retinitis pigmentosa (RP) and age-related-macular-degeneration (ARMD; 2,3). Mouse models are appropriate tools to understand the genetic and biochemical mechanisms of ocular disorders. There is a rapid increasing number of mouse mutants available suffering from various types of eye diseases (4).
The German Mouse Clinic (GMC) offers a large-scale, standardized phenotyping analysis of mouse mutants from various sources (e.g. transgenes, knockout mice) to ensure an efficient, reliable and comprehensive analysis of mouse mutants. The Eye Screen within the GMC is characterizing all incoming mice with respect to their vision, understanding the eye as an own complex sense organ. Various non-invasive methods are used to characterize the eyes in vivo. In addition, a histological analysis can be done. In the primary screen, slit lamp biomicroscopy (5) is used to recognize morphological alterations at the anterior part of the eye (mainly cornea and lens); it is extended by fundoscopy for the investigation of the posterior part of the eye (retina surface and nerve head). To determine the eye size (axial length) the laser interference biometry is used (6). If alterations were found in the primary screen, electroretinography (ERG)7 as a functional test of the retina and a histological analysis of the eye will be performed in a secondary screen.
Results
Since the onset of the German Mouse Clinic (GMC), 37 mutant mouse lines and 6 inbreeding or hybrid lines for the primary screen were provided by the Core Facility.
The eye screen started with the setup of a screening method for retinal function using a high throughput electroretinography (ERG). Out of a range of 14 different illumination intensities two luminance levels (500 and 12,500 cd/m²) were chosen for screening purposes. At 500 cd/m2 a well discernable b-wave amplitude (nearly no a-wave) mainly stemming from the rod system is induced, while light pulses at 12,500 cd/m2 induce a maximally developed b-wave response and an a-wave, coming presumably from rods and cones. Wild type baselines were established for several mouse strains (101, 129/SvJ, AKR, BALB/c, C57BL/6, CD-1, CBA, DBA, JF1); as a positive control for hereditary retinal degeneration we used C3HeB/FeJ mice. During these investigations, several animals lacking an appropriate ERG response (Fig.1B) were identified among the 129/SvJ inbred mice. Histological and molecular analysis proved the same retroviral insertion in the Pde6b gene, which is known from C3H mice (Fig. 1A)7. An associated nonsense mutation in the Pde6b gene, coding for the ß-subunit of phosphodiesterase, causes the early degeneration of the outer retina and the complete loss of rods by the age of 35 days (Fig. 1C). Several common common mouse strains, like C3H, SWR and FVB, are homozygous carriers of this recessive mutation (Pde6brd1). In the primary screen of the GMC workflow 10 mouse lines were examined, that displayed retinal degeneration caused by the Pde6brd1 allele, present in the genetic background of the mice. In one mouse line the association of microphthalmia with a gene mutation was confirmed. A new phenotype was observed in one mouse line showing a disturbed retinal lamination.
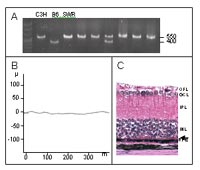
A. PCR analysis for Pde6brd1 (modified according to Gimenez and Montoliu8). Lanes 1-3 controls of a C3H, C57BL/6 and a SWR mouse, lanes 4-9 DNA of different mice to be tested for the Pde6brd1 allele.
B. A typical ERG response of a C3H mouse.
C. Histology of the retina of a C3H mouse. The arrow indicates the missing retinal layers, especially the photoreceptor cells. OFL, outer fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; RPE, retinal pigment epithel.
In the beginning of this year (2005) the primary screen was changed. The ERG method was replaced by fundoscopy. Alterations of the retina can be observed in the retinal fundus in the majority of cases. In addition, laser interference biometry was established to measure the axial length (eye size). ERG now is used in the secondary screen, to examine mice with a retinal impairment in more detail. Baseline data for the retina fundus and the axial length, so far are available for the strains C57BL/6J, C3HeB/FeJ and BALB/cByJ.
In the Eye Module of the GMC the offspring of ENU-treated C57BL/6J males are screened for an eye phenotype. By the end of 2004 more than 1000 offspring were examined by slit lamp biomicroscopy and ERG for dominant mutations. No variation or mutation leading to an eye phenotypes could be confirmed. Additionally, a recessive screen was started, where the mice are examined by slit lamp biomicroscopy and fundoscopy. Up to 500 offspring (G3) of the backcross generation of the first generation of ENU-treated C57BL/6J males were tested. One putative mutant was detected in the recessive ENU screen; confirmation cross is in progress.
Mouse line Aey20
Among our CD-1 (outbred) breeding colony several mice with retinal impairment were found. A corresponding line could be established and referred to as Aey20. It is characterised by a recessive manner of inheritance, a variable ERG and a progressive phenotype. While mapping this mutant gene we faced some problems concerning the genetic heterogeneity due to the outbred background of this line. Preliminary mapping results of Aey20 were obtained after the 2nd generation (G2) of inbreeding assigning the mutation to chromosomes 6, 7 and 19. Further detailed mapping of this line at a higher degree of inbreeding (G5) confirmed linkage to chromosome 7, with a critical region covering more than 4 Mb.
A new model for retinoschisis
A mouse line (44TNJ) with an aberrant retinal phenotype was received from an ENU-based mutagenesis screen of the Tennessee Mouse Genome Consortium, USA. The 44TNJ mutant mice are characterised by a reduced ERG responses of both a- and b-waves. Histology shows a disruption of the lamination of the retina, particularly of the outer nuclear layer, and holes in the inner nuclear layer (arrow, Fig. 2).
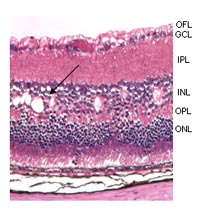
OFL – outer fiber layer, GCL – ganglion cell layer,
IPL – inner plexiforme layer, INL – inner nuclear layer,
OPL – outer plexiforme layer, ONL – outer nuclear layer.
Mapping analysis revealed linkage to the X-chromosome closest to marker DXMit117. Considering the calculated recombination frequency, the retinoschisis-1 homologue gene (Rs1h) was a good candidate. The sequence analysis of the Rs1h gene revealed a point mutation (T to C) of the second base in intron 2. This T to C exchange creates a new restriction site for NlaIII, which is not present in the wild-type gene. The presence of this novel restriction site was confirmed in 5 male mice of the mutant line, however, this restriction site was not found in wild-type controls of C57BL6, C3H, BALB/c, 129 and JF1 mice (Fig. 3).

In a PCR amplification of the Rs1h cDNA from 44TNJ mutants two products were received, instead of one from wild-type controls. The sequence analysis showed that one product from mutants contains a 10 bp insertion, identical to the first bases of intron 2, except a T to C change of the second base. Sequencing of the other product revealed a 26 bp deletion of the 3´ end of exon 2. The 44TNJ mice are a new model for retinoschisis9.
Outlook
The primary screen of the GMC will go on and all incoming mice provided by the core facility will be characterized with respect to their vision. Additionally, more offspring of ENU-treated C57BL/6J males will be examined for dominant and recessive mutations leading to an abnormal eye phenotype. The fine mapping of the Aey20 mutation is continued. The recently established mouse line rei1, with a lowered ERG response, was discovered in our JF-1 breeding colony. This mouse line and other mutants will be molecularly characterized.
Lit.: 1. Johnson G.J. and Foster A. Prevalence, incidence and distribution of visual impairment. In: G.J. Johnson, D.C. Minassian, R.A. Weale, S.K. West (eds.): The epidemiology of the eye disease. Arnold, London, UK, 2003, 3-28. 2. Rivolta et al. Retinitis pigmentosa and allied diseases: numerous diseases, genes, and inheritance patterns. Hum Mol Genet. 2002 May 15; 11(10):1219-27. 3. Stone et al. Molecular genetics of age-related macular degeneration. Hum Mol Genet. 2001 Oct 1;10(20):2285-92. 4. Graw J. The genetic and molecular basis of congenital eye defects. Nat Rev Genet. 2003 Nov; 4(11):876-88. 5. Favor J. A comparison of the dominant cataract and recessive specific-locus mutation rates induced by treatment of male mice with ethylnitrosourea. Mutat Res. 1983 Aug; 110(2):367-82. 6. Schmucker C. and Schaeffel F. In vivo biometry in the mouse eye with low coherence interferometry. Vision Res. 2004; 44(21):2445-56. 7. Dalke C. et al. Electroretinography as a screening method for mutations causing retinal dysfunction in mice. Invest Ophthalmol Vis Sci. 2004 Feb; 45(2): 601-9. 8. Gimenez E. and Montoliu M. A simple polymerase chain reaction assay for genotyping the retinal degeneration mutation (Pdeb(rd1)) in FVB/N-derived transgenic mice. Lab Anim. 2001 Apr;35(2):153-6. 9. Jablonski M., Dalke C. et al. An ENU induced mutation in Rs1h causes disruption of retinal structure and function. Mol Vis. 2005 Jul 27; 11: 569-81.


