Introduction
In tumor patients an imbalance between inhibitory and stimulatory factors exists that favors the formation of malignant cells. Soluble factors and inhibitory cytokines, e.g. TGF-ß and IL-10, have been linked to the altered immune response in tumor patients, while their cellular counterparts have not yet been described. Regulatory T cells (Treg cells) are a newly emerging T cell population that is crucial in the modulation of the immune response. Treg cells have been shown to be important for the balance of tolerance and auto-immunity. In autoimmunity a decrease of Treg cells correlates with the severity of the disease while an increase is associated with milder symptoms. Similarly, the occurrence of chronic Graft-versus-Host disease after allogeneic stem-cell transplantation is closely related with the frequency of Treg cells.
In cancer patients there is growing evidence that the number and function of these cells is altered and thereby negatively regulates the immune system in its effort to attack the tumor. For solid tumors an increase of Treg cells has been described and could be correlated to a decrease in survival. However, in hematological malignancies the frequency and function of Treg cells has not been fully characterized.
Although the importance of regulatory T cells is well established their phenotypical definition is difficult. The CD4+ CD25high T cell subset harbors a significant group of Treg cells but CD25 is also present on activated T cells and not a truly unique marker for this T-cell subpopulation. We have therefore initiated a program to molecularly define Treg cells in cancer patients to facilitate the characterization of Treg cells in future studies.
Recently, the induction of Treg cells has been linked to tolerogenic dendritic cells (DC). So far, DC have been mainly described as the most potent human antigen-presenting cells trafficking through the periphery and responsible for the induction of immunity. However, the induction of indoleamine 2,3-dioxygenase (IDO) in DC leads to decreased tryptophan in their proximity and modulates their activation status. Under these terms DC induce T cell inhibition rather than activation.
Prostaglandine E2 (PGE2), secreted by a large number of tumors, has been known as a suppressor of T cell activation. New reports show a direct association with the induction of Treg cells as well the modulation of immune responses.
Results
Regulatory T cells
Assessing B cell chronic lymphocytic leukaemia (CLL) as a model for hematological malignancies we demonstrated significantly increased frequencies of CTLA4+ FOXP3+ GITR+ CD62L+ TGF-ß1+ IL-10+ Treg cells in patients with CLL correlating with the stage of the disease. Normal regulatory function of Treg cells in CLL patients never treated with fludarabine was observed while in the majority of CLL patients treated with fludarabine the inhibitory function of Treg cells was decreased or even abrogated. In addition, frequencies of Treg cells were significantly decreased in these patients. First in vitro experiments demonstrated a preferential induction of apoptosis in CD4+ CD25+ T cells after incubation with fludarabine. We have extended these findings to patients with multiple myeloma (MM) and were able to show an increased number of fully functional Treg cells in these patients and are currently initiating studies in patients with solid tumors.
Interestingly, we could describe for the first time an increased frequency of human naïve Treg cells in adult patients with MM as well as CLL, further underlining the phenotypical and functional differences between healthy individuals and tumor patients.
Since a truly unique marker for Treg cells is still elusive, we chose genome wide expression analysis of CD4+ CD25high Treg cells and CD4+ CD25- T cells to identify novel Treg cell associated genes. CD4+ CD25high T cells and CD4+ CD25- T cells from several CLL patients and healthy individuals were isolated by FACS sorting and used to perform genome wide expression analysis using the whole genome Illumina Sentrix® Human-6 Expression BeadChip.
Even when applying very stringent filtering criteria we identified genes that were significantly different between CD4+ CD25- and CD4+ CD25high T cells. As an important control, we found genes related to Treg cells to be significantly increased in the CD4+ CD25high T cell population. These included FOXP3, which has been described as a lineage marker for Treg cells in mice and in humans, as well as GITR and CTLA4, which have also been closely related to Treg cell function.
Cluster analysis revealed that the CD4+ CD25- and CD4+ CD25high T cells from healthy individuals cluster separately and that CD4+ CD25high T cells from healthy individuals and CLL patients show differentially expressed genes (Fig 1).
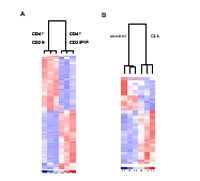
Using pathway analysis groups of genes have been identified that behave similarly to FOXP3, CTLA4, and GITR (Fig 2 and 3).

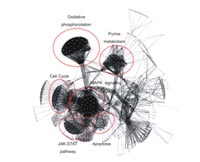
These genes are currently validated as potential candidate genes for a specific Treg cell marker.
Tolerogenic DC can induce regulatory T cells
PGE2 has recently been linked with the induction of regulatory T cells. By using genomic approaches we have been able to uncover some of the mechanisms that are involved in PGE2 mediated induction of regulatory T cells.
By genome wide gene expression profiling we show that PGE2 mediates induction of indoleamine-2,3 deoxygenase (IDO) in DC and leads to an upregulation and concomitant secretion of CD25. For both factors alone, an inhibition of normal immune responses could be shown and their combination further enhances these effects. In contrast to normally matured DC supernatants from DC matured in the presence of PGE2 show decreased stimulatory capacity of CD4+ T cells.
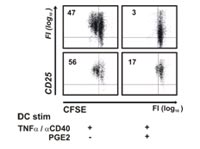
This effect is dependent on both factors as blocking of either mechanism only leads to a partial reconstitution of the T cell stimulation.
PGE2 mediated direct effects on T cells
By genome-wide transcription profiling we also attempted to elucidate direct inhibitory mechanisms of PGE2 on T cells. T cel cells were stimulated by signaling through the T cell receptor (TCR) and CD28.
Incubation with PGE2 completely abolished T cell proliferation. This inhibition was induced by an interference of PGE2 with an early step of TCR signaling as demonstrated by an inactivation of important molecules for TCR mediated proliferation, e.g. lck and ZAP70 (Fig 5).
Among the genes, which escaped PGE2 induced inhibition, we identified a number of anti-apoptotic genes. Interestingly, upregulation of these genes was associated with a partial protection of PGE2-stimulated T cells from apoptosis.
Hodgkin’s lymphoma is characterized by an enormous accumulation of immune cells at tumor sites. However, these cells lack the capacity to mount an efficient anti-tumor immune response. PGE2 is one of the main factors released by the infiltrating macrophages.
Gene expression profiling of tumor-infiltrating T cells from patients with Hodgkin’s lymphoma revealed a similar expression pattern to PGE2 treated T cells. Taken together these two mechanisms might explain the absence of an anti-tumor response in these patients while an accumulation of senescent inefficient T cells in the tumor microenvironment occurs.
Outlook
As human tumors seam to be associated with an overall increase of Treg cells, the exact characterization of the underlying mechanism as well as the exact cause of this increase are warranted. Identifying new molecular targets on Treg cells will not only help us to further portray these cells and understand their importance in human malignancies but also guide us to new targets for therapeutic interventions by either reducing Treg cell numbers or their functions.
PGE2 skews DC to a more tolerogenic phenotype, leads to the induction of impaired T cells and has inhibiting effects on T cell activation and proliferation. Furthermore, it can lead to the induction of T cells with a regulatory phenotype either via tolerogenic DC or by converting conventional T cells to Treg cells. We will need to study the effects of PGE2 treated DC on T cells to determine in which state of differentiation the T cells are stopped. Further on, it will be important to determine, how we can overcome this immune inhibition, as it might place a central role within tumor-host interactions. We strongly believe that it will be critical to understand and overcome these barriers to develop clinically efficient cancer immunotherapies such as cancer vaccines.
Lit.: 1. Beyer M et al. Reduced frequencies and suppressive function of CD4+ CD25high regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005 Sep 15;106(6). 2. von Bergwelt-Baildon MS et al. Concomitant induction of indoleamine 2,3-dioxygenase and soluble CD25 by prostaglandin E2 in human dendritic cells inhibits T cell responses. Submitted. 3. Chemnitz JM et al. Prostaglandin E2 impairs CD4+ T cell activation by inhibition of lck – implications in Hodgkin´s lymphoma. Submitted.


