Introduction
Proteomics
Most surveys utilizing high-throughput profiling of the common childhood tumor neuroblastoma conducted to date have dealt with measurements at the RNA level in order to obtain global transcriptomal signatures. Expression profiling has led to the identification of genes involved in various signaling pathways, including the MYCN, the Fyn, and the Trk pathways (1-3). Although the information gleaned from these efforts about neuroblastoma gene expression was tremendous, studies describing the protein equivalent of the transcriptome are urgently needed for several reasons. Firstly, expression profiles at the mRNA level do not necessarily predict the expression levels of proteins. Genes may be mutated to produce transcripts that are incomplete, while other mRNA transcripts may not be translated at all. Secondly, post-translational modifications, such as phosphorylation or glycosylation, cause most mRNA transcripts to give rise to more than one protein with distinctly different functional qualities. Thirdly, it is not enough that a protein is present in the cell to be functional, the protein must also reside in the correct location or cellular compartment to carry out its function.
The global analysis of cellular proteins has recently been termed “Proteomics”, the “study of all the protein forms expressed within an organism as a function of time, age, state, external factors, etc” (4). Hence proteomic studies are not only supplemental to RNA-based studies, but rather reflect the true activity status of a cell. As most drug targets are proteins, identification of drug candidates by implementing proteomics platforms for target discovery is a straightforward approach. For instance, the analysis of serum from patients with relapsed disease versus serum from patients without evidence for disease progression may be useful in tracking new protein targets as candidate drugs for the treatment of recurrent neuroblastoma. In the first part of our project we therefore aim to
(1.) Subclassify neuroblastoma and predict prognosis by serum proteomics (SELDI)
(2.) Identify neuroblastoma target proteins bei serum proteomics (2D-LC)
(3.) Validate identified target molecules on cellular level using tissue microarrays
Methylation
More recently, it has also become evident that epigenetic factors may alter expression patterns of multiple genes with potential relevance for neuroblastoma progression. It is thus likely, that a combination of events including gene translocation and rearrangement as well as altered methylation of crucial genes contributes to neuroblastoma development and progression and might be responsible for the clinical and biological heterogeneity of this tumor. CpG-island hypermethylation of gene promoters is a frequent mechanism for functional inactivation of relevant tumor-associated genes. In neuroblastoma, this mode of inactivation has been demonstrated for several genes, including caspase-8, the four TRAIL apoptosis receptors, the caspase-8 inhibitor FLIP, the RASSF1A tumor suppressor, RB1, CD44 and p16INK4a. As many of these genes are involved in apoptotic signaling and therapy responsiveness, gene hypermethylation might be a major event leading to therapy resistance of neuroblastoma cells. Analysis of epigenetic phenomena such as gene hypermethylation is therefore essential for the complete understanding of gene regulation and the biology of neuroblastoma. In the second part of our project we therefore aim to
(4.) Identify differentially methylated genes relevant for therapy resistance of neuroblastoma
(5.) Evaluate methylated candidate genes by expression, mutation and functional analysis
Project Status
Proteomics
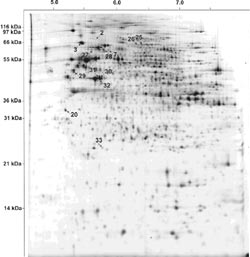
Monitoring protein expression in body fluids without previous enrichment or analytical separation became experimentally feasible by the introduction of SELDI (surface enhanced laser desorption/ionization) protein chip technology. Samples are directly applied to spots on the protein array in a volume ranging from a few microliters up to 0.5 ml. In addition to traditional MALDI (matrix assisted laser desorption/ionization), SELDI surfaces directly interact with the sample upon application. Different types of arrays with hydrophobic, hydrophilic, cation- or anion-exchanging surfaces as well as metal ion affinity surfaces are available. Unbound proteins are washed from the protein array and the protein chips are then analyzed by time-of-flight mass spectrometry, thus enabling the system to accurately determine masses of proteins present in the sample.
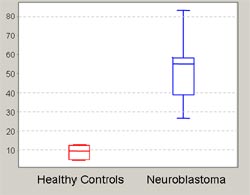
SELDI has been proven to be powerful in the detection of protein spectra characteristic for either the normal or diseased state of different cancers including breast (5) and prostate cancer (6) as well as neurodegenerative diseases (7-9). Presently, sera of neuroblastoma patients are routinely analyzed for catecholamine levels, since overproduction of noradrenaline pathway metabolites is one hallmark of neuroblastic cells. We have conducted a preliminary analysis on neuroblastoma serum samples using the SELDI system to ensure reproducibility of the protein spectra. These preliminary results demonstrate the suitability of routinely collected sera without pretreatment for SELDI analysis. Serum samples from > 70 neuroblastoma patients (corresponding to a tumor panel previously analyzed by Affymetrix microarrays) and > 70 age- and sex-matched controls are available for comprehensive analysis in this project. We use the recently introduced ProteinChip System Series 4000 (Ciphergen, Göttingen) to analyze the serum proteomes of neuroblastoma patients in comparison to patients with other childhood cancers and healthy control children. A pilot analysis of serum samples from 10 stage 4 neuroblastoma patients and 10 age- and gender-matched healthy children was carried out in order to identify optimal chip surfaces and serum pre-treatment methods. Fig. 3 shows an example of differentially represented serum protein peaks.
Methylation
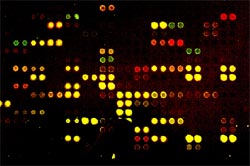
To identify differentially methylated genes after development of chemotherapy resistance, we here use a microarray-based technology (DMH) in collaboration with the „SMP Epigenetics“ (A. Waha, University of Bonn). This technology currently allows simultaneous assessment of methylation changes within 7680 CpG-rich fragments of the human genome. Two-color labeling with Cy dyes allows for the identification of differentially methylated DNA regions. The sequence information can be used to investigate de novo methylation of identified target genes in bisulfite-treated genomic DNA from primary tumor samples.
The aim of this project is to identify gene sets with aberrant methylation involved in therapy resistance and progression of neuroblastoma using array-based genome-wide methylation analyses 1. in a neuroblastoma cell culture model and 2. in primary neuroblastomas. To this end, we are investigating methylation profiles in a preclinical model of drug resistance in neuroblastoma. For this purpose, cell lines with specific resistance to cisplatin (10-fold), doxorubicin (30-fold) and etoposide (100-fold) have been developed by long-term cytotoxic treatment and are analyzed in comparison to the chemotherapy-sensitive parental LAN1 human neuroblastoma cell line. Global expression profiles of the resistant cell lines in comparison to the parental cells have been obtained by oligonucleotide arrays (Affymetrix Inc). Hypermethylation of gene promoter regions is associated with transcriptional inactivation of tumor suppressor genes, whereas hypomethylation of oncogenes promotes their expression. We identified several hyper- or hypomethylated regions in resistant versus sensitive cells. Novel, previously unidentified candidate genes for therapy resistance are currently being validated by MS-PCR and bisulphite-sequencing in neuroblastoma cell lines as well as primary tumors (initial diagnosis versus relapse specimens).
Outlook
SELDI technology will be a straightforward approach to answer the following questions. (1) Can we identify disease specific protein signatures for neuroblastoma patients? (2) Can we monitor differences in sera from patients with relapse versus event-free survivors which may be predictive for recurrence of disease? (3) Can we distinguish between protein patterns from patients with stage 4 and stage 4s neuroblastomas, enabling us to better guage the aggressivity of the tumor? (4) Are changes in serum composition following treatment detectable and informative with respect to treatment response? The final goal of our proteomic study is to define predictive patterns for a priori assignment of patients to different risk groups, and to monitor these patterns prospectively.
Our genome wide methylation approach will unmask new genes affected by hypermethylation or hypomethylation in resistant neuroblastoma cells that may be relevant for better prediction of disease outcome, but may also provide new targets for therapeutic approaches. The whole study aims at the identification of molecules beyond gene expression levels, in particular proteins and epigenetic factors. The results will contribute to a precise molecular classification of neuroblastoma and a reliable definition of predictive signatures. The long-term goal is the improved understanding of molecular mechanisms contributing to the development and progression of cancer, finally leading to more specific and successful therapies.
Lit.: 1. Alaminos, M et al. Genome-wide analysis of gene expression associated with MYCN in human neuroblastoma, Cancer Res. 63: 4538-46, 2003. 2. Berwanger, B et al. Loss of a FYN-regulated differentiation and growth arrest pathway in advanced stage neuroblastoma, Cancer Cell. 2: 377-86, 2002. 3. Schulte, JH et al. Neurotrophin-receptor expression rather than ligand-induced activation contributes to the transcriptomal program of neuroblastoma cells., Cancer Res. conditionally accepted:, 2004. 4. Reynolds, T. For proteomics research, a new race has begun, J Natl Cancer Inst. 94: 552-4, 2002. 5. Wulfkuhle, JD et al. New approaches to proteomic analysis of breast cancer, Proteomics. 1: 1205-15, 2001. 6. Petricoin, EF et al. Clinical proteomics: translating benchside promise into bedside reality, Nat Rev Drug Discov. 1: 683-95, 2002. 7. Lewczuk, P t al. The amyloid-beta (Abeta) peptide pattern in cerebrospinal fluid in Alzheimer's disease: evidence of a novel carboxyterminally elongated Abeta peptide, Rapid Commun Mass Spectrom. 17: 1291-6, 2003. 8. Davies, H et al Profiling of amyloid beta peptide variants using SELDI Protein Chip arrays, Biotechniques. 27: 1258-61, 1999. 9. Goldstein, LE et al Cytosolic beta-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer's disease, Lancet. 361: 1258-65, 2003. 10. Chong, BE et al. Chromatofocusing nonporous reversed-phase high-performance liquid chromatography/ electrospray ionization time-of-flight mass spectrometry of proteins from human breast cancer whole cell lysates: a novel two-dimensional liquid chromatography/mass spectrometry method, Rapid Commun Mass Spectrom. 15: 291-6, 2001. 11. Zheng, S et al.Two-dimensional liquid chromatography protein expression mapping for differential proteomic analysis of normal and O157:H7 Escherichia coli, Biotechniques. 35: 1202-12, 2003.


