Introduction
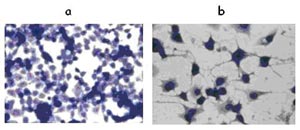
Neuroblastomas belong to the group of embryonal childhood tumors. They apparently originate from early progenitor cells in which the normal genetic differentiation programs have been disrupted resulting in a differentiation block and a compensatory and inadequate proliferation of the transformed progenitor cells. Interestingly, the neuroblastomas exhibit the highest frequency of spontaneous regression and differentiation of all malignancies known. We hypothesize that a differentiation program of neuroblastoma cells can be pharmacologically reactivated eventually resulting in the differentiation of the malignant tumor cells towards normal ganglionic cells. Our previous work has demonstrated that histone-deacetylase (HDAC)-inhibitors are able to reactivate such a differentiation process (see figure 1). So far, the genes, which regulate this differentiation program have not been identified. Their definition and pharmacological activation could present a novel strategy to improve the poor outcome of neuroblastomas and other childhood malignancies.
Using microarray analysis, we have identified a number of potential regulatory genes, controlling a differentiation program in neuroblastoma cells. The major aims of the project are 1. to functionally characterize these differentiation control genes and 2. to identify novel compounds for differentiation therapy of neuroblastomas.
Project Status
Functional screening of candidate genes
Using an high throughput RNA-interference approach, we seek to determine the function of 112 identified candidate genes in the context of neuroblastoma differentiation. These genes are activated within hours upon initiation of the differentiation process or are expressed in pharmacologically differentiated neuroblastoma cells as well as in normal adrenal medulla as a reference tissue for terminally differentiated normal sympathetic ganglionic tissue.
In colloboration with Cenix Biosciences, Dresden, a protocol useful for 96well format transfection, morphological read-out, and determination of cell proliferation was established. Furthermore, siRNAs against the 112 candidate genes have been designed and functionally tested via qRT-PCR. Because some of the siRNAs did not show the required knock-down efficacy of at least 70%, a second round of siRNA design and functional evaluation is in progress. Interestingly, knock-down of one candidate gene revealed induction of morphological differentiation in preliminary experiments.
Compounds
So far, we have tested a small series of HDAC-inhibitors with respect to their potency for inducing differentiation of neuroblastoma cells. We have identified an interesting compound acting at nanomolar concentrations. This means, that on a molar basis, this compound is 103 to 106 fold more potent compared with so far described HDAC-inhibitors with respect to their differentiation inducing potential in neuroblastoma cells. The compound strongly inhibits cell proliferation in MYCN single copy as well as MYCN amplified cells. The toxicity on normal, not transformed cells is currently under investigation.
Outlook
We will re-design non-functional siRNAs against our candidate differentiation genes in collaboration with Cenix-Biosciences. After having completed siRNA validation, all 112 candidate genes will be knocked-down and the effect on proliferation, morphological differentiation and induction of marker gene expression will be evaluated. Genes identified in this screen will be further characterized by overexpression in neuroblastoma cells in vitro. The consequence on the tumor biology for neuroblastoma cells will be further evaluated in a mouse model. These genes will then be used for screening of a compound library in order to identify novel substances with a potential in differentiation therapy of neuroblastoma. In parallel, we will further characterize the novel identified HDAC-inhibitor for its potential use in differentiation therapy by determining its toxicity on normal cells and in a neuroblastoma mouse model.


