Introduction
Unipolar depression (UPD) has a strong genetic component, with heritabilities of up to 70%. Association studies for UPD rely on candidate genes, which are chosen from the current concepts of the pathophysiology of UPD. These association studies based on the current pathophysiological hypothesis of UPD have yielded only controversial, rarely replicated results. Therefore, a search for novel candidate genes using an unbiased, genome-wide approach appears to be more promising. It is our goal to identify candidate genes using hypothesis-free proteome and transcriptome strategies including proteomics analyses of the cerebrospinal fluid (CSF) of patients with UPD and controls and gene expression analysis in animal models of depression and after antidepressant treatment. Identified candidate genes will then be tested for association in 1,000 patients and 1,000 healthy controls by typing dense sets of SNPs as genetic markers. Any significant SNP association will be replicated in independent samples (project 8.3.4) and functionally validated in cell culture and in mouse models.
Our project builds on findings, which were raised by three different approaches:
Project Status
Proteomics
CSF-analyses have shown that the protein patterns in CSF are very complex with a few predominant proteins such as serum proteins. However, many other proteins, whose identity remains unknown, are present in small quantities and require sophisticated methods for their identification. To this end we have focused our studies on the development of suitable CSF sample preparation methods that are compatible with subsequent proteomic analyses. The removal of major proteins, in particular human serum albumin and immunoglobulins, turned out to be critical for subsequent analytical procedures. In order to prevent the loss of potentially important CSF marker proteins we have obtained a more specific sample preparation resin that is made up of monoclonal antibodies against six major proteins found in serum and CSF (albumin, transferrin, IgG, haptoglobin, antitrypsin, and IgA). This resin is now routinely used for our CSF sample preparation and subsequent proteomic analysis. To extent our comparative CSF proteome studies we have also implemented the so-called shotgun mass spectrometry analysis approach using CSF protein digests. This type of analysis results in a greater depth of protein identifications as compared to the traditional 2-D gel electrophoresis methodology. In particular, low abundant proteins can be identified in the presence of high abundant proteins. To this end we have been able to identify over one hundred proteins using a test sample from a patient with normal pressure hydrocephalus.
Animal models and expression profiling
A host of data implicates central and peripheral disturbances of hypothalamic-pituitary-adrenocortical (HPA-) axis regulation in the pathogenesis of depression, especially a dysregulation of the hypothalamic peptide CRH and normalization of these defects as a prerequisite of clinical response to medication1. The behavioral analyses of conventional Crhr1 null mutants 2 are hampered by the fact that these mice display severe glucocorticoid deficiency, confounding the interpretation of behavioral data. To selectively dissect CRH/Crhr1 central nervous system pathways modulating behavior from those regulating neuroendocrine function, we generated a conditional Crhr1 knockout using the Cre/loxP system driving Cre recombinase expression by a Calcium Calmodulin-kinase IIalpha (CaMKIIalpha) promotor. In these animals Crhr1 function is inactivated postnatally in forebrain and limbic brain structures while sparing hypothalamic and pituitary expression sites, so as to leave the HPA-system regulation intact 3. We further characterized the two inbred mouse strains C57BL/6 and DBA/2, which have been suggested to represent opposite extremes in terms of emotionality. The anxiety-like behavior of DBA/2 mice could be reversed by administration of the antidepressant paroxetin for 28 days, but not shorter treatment periods. The antidepressant treatment did not have any behavioral effects in the non-anxious C57BL/6 mice, making the two strains ideal models for the identification of genes relevant for the behavioral response to antidepressant drugs. During the past years a microarray facility has been established at the MPI of Psychiatry. The actual MPI 22k mouse array contains approximately 22,000 GenBank clones, which in turn – due to a certain extent of redundancy – represent 18,000 unique UniGene clusters. Optimized standard procedures for template generation, labeling, hybridization and data acquisition ensure reproducible results. Differences in gene expression will be investigated in a brain region specific manner (5 brain regions) using microdissection and micro-punch techniques.
Human Genetics
Genes identified by the above mentioned approaches – proteomics and gene expression analysis – will be tested for association in a sample of 1,000 patients with UPD and 1,000 controls, recruited in part within NGFN-1 using SNPs as genetic markers. Cases are inpatients from our Institute and psychiatric hospitals in Augsburg and Ingolstadt. Patients are diagnosed according to DSM IV using the Schedule for Clinical Assessment in Neuropsychiatry (SCAN) by WHO-certified raters. SNPs will be selected from public and private databases. After a first screening phase using about 1 SNP/5-10kb as well as tagging SNPs, additional SNPs will be genotyped in genes showing association and their neighboring genes to construct linkage disequilibrium (LD) maps. This step will be followed by typing of a replication sample (see project 8.3.4). In addition, samples with bipolar disorder and schizophrenia will be analyzed to define the biological relevance of the identified disease genes (projects 8.3.2, 8.3.5, and 8.3.6).
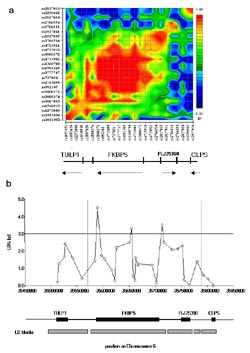
In a first approach we genotyped 34 candidate genes related to the regulation of the HPA-axis in 740 depressed patients and 550 healthy controls and failed to observe any significant case/control associations. We could, however, identify an association of SNPs in FKBP5, a glucocorticoid receptor-regulating co-chaperone of hsp90 with response to antidepressant treatment (OR: 28.0; p = 5.5x10-6) 4. We analyzed 57 single SNPs and haplotypes for an association with parameters related to the response to antidepressant treatment after 2 and 5 weeks of hospitalization and to remission at discharge (N=233). We found significant associations between three SNPs (rs1360780; rs1334894 and rs755658) in the FKBP5 gene and response for at least two of the three time points. We therefore genotyped an additional 27 SNPs in 288 kb around FKBP5 including the neighboring genes TULP1, FLJ25390 and CLPS for which we constructed a linkage disequilibirium (LD) block map based on D'. (Fig. 1a). For the association with response at 2 weeks, the smallest p-values were found with SNPs within the major block of LD containing FKBP5 (Fig. 1b).
Here we observed 3 peaks of strong association, one in the putative promoter region (rs4713916, p=0.00031), the second in intron 2 (rs1360780, p=0.00048) and the third in the 3’ untranslated region (rs3800373, p=0.00003). A similar pattern of association was found with response at 5 weeks and remission. This finding could be replicated in an independent patient sample from two Bavarian hospitals.
These SNPs in the FKBP5 gene were also associated with a three-fold higher number of previous depressive episodes.
Both, faster response to antidepressants and more frequent depressive episodes could be related to innate differences in HPA-axis regulation. A series of in vivo and in vitro studies have implicated FKBP5 as important regulator of GR sensitivity, and thus HPA-axis responsivity. The hsp90 co-chaperone FKBP5 is part of the mature GR heterocomplex. Upon hormone binding, FKBP5 is replaced by FKBP4, which then recruits dynein into the complex, allowing its nuclear translocation and transcriptional activity. We found that in vitro overexpression of human FKBP5 reduces hormone binding affinity and nuclear translocation of GR (Wochnik et al., unpublished data). A naturally occurring overexpression of FKBP5 causes GR insensitivity in New World Monkeys, which is accompanied by increased plasma cortisol levels. Furthermore, glucocorticoids induce FKBP5 expression as part of an intracellular ultra-short negative feedback loop for GR activity. To investigate possible functional effects of FKBP5 polymorphisms, we assessed FKBP5 protein levels in lymphocytes. Lymphocytes of TT homozygotes for rs1360780, the genotype that was associated with faster response to antidepressants, had FKBP5 levels twice as high as those of individuals with the two other genotypes (p=0.024). In contrast to the situation observed in New World Monkeys, this increase in FKBP5 expression was not accompanied by enhanced circulating cortisol levels.
To identify the mechanism of increased FKBP5 protein levels, we quantified FKBP5 mRNA levels. There were no direct effects of the rs1360780 genotype on mRNA levels in peripheral blood monocytes (PBMCs) of healthy probands, suggesting that not increased transcription but enhanced translation or protein stability contribute to increased FKBP5 levels. We did, however, observe genotype-dependent differences in the positive correlation of plasma cortisol and FKBP5 mRNA levels. A much stronger correlation was observed in TT homozygotes of rs1360780, which was significantly different (p=0.012) from the other genotypes. This suggests that in TT homozygotes GR signaling and HPA-axis reactivity are more tightly controlled by the reported interaction between GR and FKBP5. This could be due to an adaptively altered composition of the GR-chaperone heterocomplex that would lead to an increased sensitivity even to smaller changes in FKBP5 expression.
If this were true, one would expect FKBP5 genotype-dependent differences in the neuroendocrine abnormalities observed in depressed patients. We noticed a significantly lower ACTH response in the combined dexamethasone-suppression/CRH-stimulation (Dex/CRH) test in depressed patients carrying the TT genotype of rs1360780 compared to the other genotypes. The compensatorily activated alternate mechanisms for GR and HPA-axis regulation appear to partially counteract depression-related HPA-axis hyperactivity but not fully prevent them. These genotype-dependent differences in the Dex-CRH test also indicate that the functional effects of FKBP5 variants extend beyond peripheral blood cells to organs relevant for the pathophysiology of depression. We therefore propose that, even though T homozygotes are as severely depressed as the other patients, their HPA-axis regulation is less impaired due to compensatory mechanisms elicited by increased FKBP5, allowing a faster restoration of normal HPA-axis function.
Outlook
For the identified FKBP5 haplotype (containing the minor alleles of rs4713916 (promoter region), rs3800372 (intron 1), rs1360780 (intron 2), rs3800373 (3’UTR)) we will isolate a human BAC carrying the risk alleles and will investigate whether this has an effect on gene transcription by comparison to wildtype levels. In primary lymphocytes we will then test glucocorticoid receptor activity using a reporter fused to GRE (glucocorticoid receptor response elements). In parallel, glucocorticoid response will be tested by transfecting a human BAC clone carrying the risk haplotype. In case of altered response, we envisage to establish animal models and test for changes in stress-responsive and anxiety-like behavior. These experiments will serve as templates for the validation of the SNP associations that will be identified by proteomics and transcriptomics. The newly developed mouse models will then be behaviorally phenotyped.
Lit.: 1. Holsboer F The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000 Nov;23: 477-501. 2. Timpl P et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998 June;19: 162-6. 3. Muller MB et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003 Oct; 6:1100-07. 4. Binder E et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004 Dec;36(12):1319-25.


