Introduction
Type I interferons (IFNs) are pivotal cytokines in the early innate immune response that exhibit important antiviral, antitumor, and immunomodulatory effects. Recently, a central role for type I IFNs in lipopolysaccharide (LPS) – induced endotoxin shock was also demonstrated [KARAGHIOSOFF M, NAT IMMUNOL, 2003]. Furthermore, IFN## has been suggested to be centrally involved in the pathogenesis of lupus erythematosus, a systemic autoimmune disease that is 9 times more frequent in females than in males [BLANCO P, SCIENCE, 2001].
Plasmacytoid dendritic cells (pDCs) represent a rare population of blood cells that are able to produce extremely large amounts of type I IFN. After antigen uptake and maturation they are critical antigen presenting cells linking innate and adaptive immunity. pDCs express Toll like receptor 7 (TLR7) and TLR9, and production of type I IFN in pDCs is induced upon ligation with imidazoquinoline, an immune adjuvant, or its derivative R-848, for TLR7 and bacterial unmethylated CpG DNA for TLR9. In certain cell types, TLR7 and TLR9 signal via the MyD88-IRAK4-TRAF6 pathway leading to nuclear translocation of NF#B and the transcriptional response of cytokine genes. The pathways leading to type I IFN expression have not been completely elucidated.
Results/Project Status
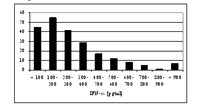
Within NGFN1 we have postulated that the study of “proximal phenotypes” may reveal insights into the functional consequences of common naturally occurring gene variants in humans: these may remain undetected by – mostly underpowered – genetic association studies employing “distal phenotypes”, e.g. clinical diseases. To test this hypothesis we have designed a highly standardized experiment: we studied the type I interferon (IFN) production of freshly-isolated human blood pDCs upon stimulation with TLR7 and TLR9 ligands. Our data indicate an enormous subject-to-subject heterogeneity of IFN-# secretion (10-1000 times; Fig. 1; [BERGHÖFER B, CLIN EXP ALLERGY, 2005]).
In contrast, the repeated stimulation of pDCs from the same individual on different occasions revealed low variability (1-3 times) suggesting a high reproducibility. Moreover, upon TLR7 stimulation, pDCs of female donors secreted fourfold higher quantities of INF-# compared to male donors (n=100; this result was confirmed by investigating a second independent group of 120 donors, p<0.0001; Fig. 2).
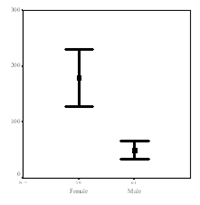
In contrast, upon TLR9 stimulation, no gender difference of IFN-# secretion was observed. Thus, the striking gender difference in the capacity to secrete INF-# is unlikely to be related to differences in the signalling cascade downstream of TLR7 and TLR9.
The TLR7 gene is located on the X chromosome, and dosage imbalance of females having two X chromosomes compared with the one X chromosome of males is normally resolved by silencing one of the X chromosomes in females early in development. However, in humans, at least 15% of genes on the X chromosome escape inactivation. Hence, we hypothesise that TLR7 belongs to this group of genes escaping silencing, explaining the observed phenotype of female hyper-responsiveness upon TLR7 stimulation.
Based on our preliminary results, we hypothesize in addition that the pronounced inter-individual differences in type I IFN production are partly due to genetic factors influencing TLR7 and/or TLR9 signalling. Interestingly, a common stop codon polymorphism in the TLR5 gene, abolishing flagellin signalling was recently associated with susceptibility to Legionnaire´s disease [HAWN TR, J EXP MED, 2003]. Inherited IRAK-4 (centrally involved in TLR signalling) deficiency in humans was associated with a markedly mild phenotype: the otherwise healthy children developed infections caused by S. pneumoniae and S. aureus, but were later in life well with no treatment [PICARD C, SCIENCE, 2003]. The authors concluded, that protective immunity to most infectious agents was conferred without the action of IL-1R, IL-18R, and TLRs. However, TLR signalling through MyD88-independent pathways is not disrupted by IRAK-4 deficiency and the signalling pathways leading to TLR7, TLR9 (and TLR8) induced type I IFN production are currently under investigation and both TLRs may signal with kinases in addition to IRAK-4.
Thus, we raise the following hypotheses:
1. Hyper-responsiveness of pDCs from females upon TLR7 stimulation is due to escape from silencing of the TLR7 gene from the inactivated X chromosome.
2. The quantitative trait “type I IFN production in pDCs upon TLR7 and TLR9 stimulation” is partly due to genetic variation of TLR7 and TLR9 genes (in males and females).
3. The capacity of human pDCs to mount an early and high type I IFN production is associated with resistance of the host against viral and/or bacterial infection.
The goals of this project are:
(i) to dissect the genetic basis of hyper-responsiveness in type I IFN production from pDCs of females upon TLR7 ligation.
(ii) to investigate the genetic association of TLR7 and TLR9 haplotypes and/or haplotype estimates with the quantitative phenotype type I IFN production in pDCs.
(iii) to investigate the association of TLR7 and TLR9 haplotypes with defined proximal phenotypes in a large cohort study of infectious diseases: network of competence for community acquired pneumonia, CAPNET, Germany
This project will reveal detailed information on the role of TLR7 and TLR9 as potential resistance factors for infections and autoimmune diseases.
Outlook
There are profound differences between females and males in the immune response including lower mortality of sepsis and community-acquired pneumonia, and higher incidence of lupus erythematosus and other autoimmune diseases in females. IFN## has been suggested to be centrally involved in the pathogenesis of lupus erythematosus. The striking difference of type I IFN release from pDCs of females compared to release of type I IFN from pDCs of males may be causally involved in the sex distribution of autoimmune and infectious diseases. The molecular dissection of the genetic basis of gender differences and of inter-individual differences in type I IFN production of human pDCs may support the development of new therapeutic strategies in infectious and autoimmune diseases with inadequate production of type I IFN.
Lit.: 1. Karaghiosoff M et al. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat Immunol. 2003 May;4(5):471-7. 2. Blanco P et al. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001 Nov 16;294(5546):1540-3. 3. Berghöfer et al. Common human Toll-like receptor 9 (TLR9) polymorphisms and haplotypes: association with atopy and functional relevance. Clin Exp Allergy, in press. 4. Hawn TR et al., A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires' disease. J Exp Med. 2003 Nov 17;198(10):1563-72. 5. Picard C et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003 Mar 28;299(5615):2076-9.


