Introduction
For decades, the activation of inflammatory responses by bacteria has interested investigators in a wide variety of infectious diseases. The mechanisms by which the host restrains the inflammatory response and restrict its temporal course is less clear.
Aberrant regulation of inflammatory responsiveness underlies the pathogenesis of widely disparate clinical syndromes such as sepsis, inflammatory bowel diseases, atherogenesis and other inflammatory disorders. During infection, macrophages recognize conserved pathogen-associated molecular patterns through the Toll-like receptor (TLR) family of proteins. Activation of TLRs triggers a rapid antimicrobial response through stimulation of pro-inflammatory pathways. The ten members of TLRs described collectively recognize a wide range of microbial components. Thus, bacterial lipoproteins, lipopolysaccharide (LPS) and DNA containing unmethylated CpG dinucleotides are recognized by TLR2, 4 and 9 respectively, whereas RNA generated during viral infections is a specific ligand for TLR3.
Although the typical host response is one of self-defense, characterized by the induction of inflammatory mediators to kill or limit dissemination of the microorganism, resident commensal and non-virulent microorganisms colonize body surfaces without induction of uncontrolled inflammation. Notably many of these bacteria have many of the molecular patterns that would induce inflammation, yet they persist in exceedingly large numbers for example in the gut without overt inflammatory responses. Thus mechanisms must exist to suppress innate immunity and inflammation at the level of the epithelial cell. Atheroclerosis is a chronic inflammatory disease as well as a disorder of chronic lipid metabolism. As modulators of both lipid metabolism and immune responses, macrophages play a central role in the atherogenic process. The accumulation of cholesterol-loaded macrophages in the arterial wall is a hallmark of the early atherosclerotic lesion. Exogenuos lipids, derived from the walls of microbial pathogens, and endogenous lipids, generated by the oxidation of fatty acids and cholesterol can both activate cells of the immune system and stimulate potent pro-inflammatory events recruiting other cells leading to the development of a complex lesion. Thus processes modulating the balance between macrophage lipid uptake and efflux would be expected to exacerbate lesion formation.
In this project, naturally occurring ligands of TLR3 and TLR4, discovered in NGFN1 are used to provide direct evidence for the cross talk between this pathway and the PPAR and LXR regulated pathways. Since enteric bacteria and listeriolysin modulate LXR and PPAR expression via the MYD88-IRAK4-TRAF6-IKK-NFkB and TRIF-IRF3-IFNR pathways respectively, we hypothesize the existence of an interface between TLR-induced pathways and that of the nuclear hormone receptor activation pathway.
Project Status
Pathway of TLR2, TLR3 and TLR4
Using cell lines transfected for the TLR2-, TLR3- and the TLR4-pathway, we find that the gram-positive pathogen L. monocytogenes induces NF-kB activation via the TLR-2 and TLR-4, but not the TLR-3 receptor. We also observed direct activation of the NFkB transcription factor independent of any of the TLRs used suggesting the presence of a TLR-independent NfkB inducing pathway. We have now inititated studies with mutants lacking defined components of L. monocytogenes in order to identify bacterial components involved in signalling via the TLR4 pathway, previously only thought to be required for LPS signalling.
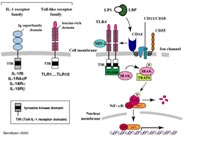
NOD-LRR-Proteins
We used cell lines transfected with recombinants expressing the NOD1 and NOD2, two nucleotide-binding-site (NBS), Leucine-Rich Repeat-containing (LRR)-containing proteins considered to be important molecules for surveying the cytoplasm of cells for intracellular pathogens, and examined for the ability of listeriolysin (LLO)-producing and non-producing mutants of Listeria to activate these molecules. We find the intracellular bacteria can induce both NOD1 and NOD2 and that intracellular activation of this pathway requires listeriolysin function. Studies on the characterizaton of signalling pathways leading to activation of NOD by intracellular bacteria are now underway.
NRH activation pathway
Expression profile analysis of epithelial cells treated with Shiga-Toxin-producing Escherichia coli (STEC) demonstrate that these have immunosuppressive properties. We have previously shown that one of the pathways used involves modification of the NFkB transcription factor that reversibly induces its translocation out of the cell nucleus. More recently we find that STEC bacteria also induced expression peroxisome proliferator-activated receptor ß (PPARß), a nuclear hormone receptor molecule that can counterregulate inflammatory responses. In ongoing studies we wish to identify the pathway leading to PPARß activation of epithelial cells by the bacteria and its role in the pathogenesis of infection.
Outlook
Our next steps comprise
• Detection of new bacterial PAMP-ligands for TLR2, TLR3 and TLR4
• Detection of bacteria causing immune suppression via NRH
• Expression profiling of cells activated and immunosuppressed by bacteria
• Analysis of TLR-signalling transduction during the modulation of the signalling transduction of the nuclear hormone receptor family (NHR)
• Analysis of NHR-effects on TLR-signalling transduction.
Lit.: 1. Hauf N., Chakraborty T. Suppression of NF-kappa B activation and proinflammatory cytokine expression by Shiga toxin-producing Escherichia coli. J. Immunol. 2003 170: 2074-82. 2. Peters C et al. Tailoring host immune responses to Listeria by manipulation of virulence genes – the interface between innate and aquired immunity. FEMS Immunol Med Microbiol. 2003 35;243-53. 3. Darji A et al.Induction of immune responses by attenuated isogenic mutant strains of Listeria monocytogenes. Vaccines. 2003 21;102-9 4. Opitz B, Püschel A, Förster S, Beermann W, Schmeck B, Chakraborty T, Suttorp N, Hippenstiel S. Nod1 mediates p38 and p42/44 MAP kinase activation and IL-8 secretion induced by intra¬cellular bacteria. J Immunol. In press, 2005 5. Mc Whirter S et al. IFN-regulatory factor 3-dependent gene expression is defective in TBK1-deficient mouse embryonic fibroblasts. PNAS. 2004 101;233-238. Figure source: iir.suite.dk/IIR/09innImm/TLR.htm.


