
Introduction
Colorectal cancer (CRC) is the second leading type of cancer and the second leading cause of cancer-related deaths in industrialized Western countries. An estimated 60000 new cases per year develop in Germany. 75 percent of the new CRCs are candidates for surgical resection aimed at cure. The long term survival of CRC patients depends on the local tumor stage and the potential for distant metastases. Neoadjuvant and adjuvant chemotherapeutic and radiotherapeutic strategies are used to prevent the locoregional and distant recurrences, but are effective only in fractions of CRCs. Chemotherapy can achieve partial remission of distant metastases but no cure. Thus, there is a demand for new diagnostic markers and therapeutic targets.
The hallmark of tumor malignancy is tumor cell invasion and distinguishes colorectal carcinoma from adenoma. The properties of invasion depend on complex functional capabilities of tumor cells which are facilitated by genetic alterations and regulated by interactions of tumor and surrounding stroma. Processes of dedifferentiation and epithelio-mesenchymal transition (EMT) of tumor cells have been demonstrated by us and others as features of invasion in CRC (1). EMT occurs during critical phases of embryonic development and is regulated by several signal-transduction pathways, e.g. Wnt-pathway, c-Met-pathway, and TGFb-signaling (2). In CRC the dysregulation of the Wnt-pathway reactivates a developmental programme leading to de¬differen¬tiation, EMT and invasion of tumor cells. The initial and most decisive genetic alteration, which is found in more than 80% of sporadic colorectal carcinomas (3), is the loss of function mutation of the APC tumor suppressor gene, leading to aberrant activation of the Wnt-pathway and its component b-catenin. The enigma is, how this dysregulation at the start of colorectal tumorigenesis causes at first the non-invasive growth of adenoma and after that the invasive growth of carcinoma. Therefore studies of intratumorous regulatory networks and tumor-stroma-interactions, that involve the Wnt-pathway and nuclear b-catenin activity, are mandatory to understand the driving forces of CRC progression.
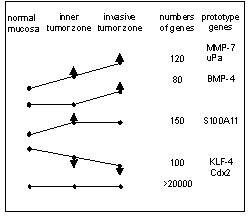 | |
| Fig 1: Five out of eight patterns of changes of RNA-expression in normal mucosa versus the inner differentiated zone versus the outer invasive zone of CRC. Shown are typical patterns of changes of expression, the number of genes showing the pattern and typical marker genes within the patterns. | |
Results
We have detected a zonal variation of nuclear b-catenin expression in CRC by demonstrating low nuclear expression in tumor cells of an inner zone, and high nuclear expressions in the tumor cells of an outer zone of CRC. Low nuclear b-catenin expression was associated with differentiation, tubule formation and high proliferation of tumor cells of CRC, similar to adenoma. High nuclear b-catenin expression was correla¬ted with dedifferentiation, low proliferation, EMT and inva¬sive¬ness of tumor cells (1). We rediscovered this zonal expression of b-catenin also in lymph node metastases, where low nuclear b-catenin characterized again areas of differentiation and tubule formation of the tumor cells similar to the primary tumor (4). Since all tumor cells in an individual tumor harbour APC-mutations, a nuclear accumulation of b-catenin can not be due to this alteration alone, but its intracellular distribution within different tumor areas has to be explained by additional events. Due to the observed dynamic changes in intracellular b-catenin distribution and the tumor cell phenotype, a main driving force of these pro¬cesses must be the tumor environment acting on the genetically altered tumor cells. These findings indicate a non-linear progression of CRC and a regulation of nuclear b-catenin expression and invasive properties by the tumor environment despite the genetic alterations (5-7). By exploiting this regulatory model of invasion, we detected new b-catenin targets genes, which are involved in CRC invasion, e.g. MMP7 (8) and the g2 chain of laminin 5 (9), as well as new candidates for Wnt-regulation, e.g. p16 (10).
We applied genome-wide expression profiling by the U133A microarray (Affymetrix) to analyze the intratumorous transcriptional regulation of CRC invasion. We isolated from the same surgical specimens the normal mucosa, the inner differentiated zone and the outer invasive zone of CRC by laser-microdissection, and we established a mRNA-amplification protocol for reproducible RNA-profiles out of small microdissected tissue samples. The comparative analysis of the profiles (normal mucosa versus inner zone of CRC versus outer zone of CRC) of 11 patients revealed various patterns of up- and downregulated genes indicating different intratumorous transcriptional regulations (Fig.1). Two patterns characterize genes that are significantly upregulated at the outer invasive zone compared to the inner differentiated zone of CRC. These genes are candidates for the regulation and induction of CRC invasion and progression. Among these genes are nine out of thirty previously described b-catenin targets (e.g. MMP-7, uPA, uPA-R, BMP-4, laminin5 g2, fibronectin, CD44, etc.), confirming the methodological reliability of our study. Among upregulated invasion-associated genes, three new Wnt/b-catenin targets, MT1-MMP, uPA and tenascin-C, were identified. We validated these new targets by a functional analysis of their transcriptional activation and by their immunohistochemical detection in the outer invasive zone of CRC (11-13).
Outlook
Based on these results we are confident that our new approach to profile the intratumorous zonal expression and regu¬lation of genes is suitable to detect new relevant markers and targets for a diagnostic prediction and thera¬peutic interruption of CRC progression. The zonal in-situ-expression and localisation of key factors in tumor cells or stromal tissue will be evaluated by immunohistochemistry, in situ hybridization or quantitative real-time RT-PCR after differen¬tial laser-microdissection of either cells.
Prognostic valida¬tion of candidate factors for CRC-pro¬gression will be done by an immunohistochemical screening of CRC tissue samples of large patient cohorts with clinical follow-up data.
Functional validation of key factors for invasion, which are candidates for a transcriptional regulation by b-catenin, will be performed by reporter assays, gel shift assays and b-catenin siRNA experiments which were successfully used in our previous studies of Wnt-targets (11-13). We additionally established cell culture models to study the phenotypical and functional effect of variable nuclear b-catenin expression as well as its regulation in CRC cell lines with defined APC loss of function mutations. One model uses the SW480 cancer cells, whose dissociated or aggregated growth pattern are correlated to different nuclear b-catenin expression (4). In a second model the growth pattern and nuclear b-catenin expression of the Caco-2 cancer cells is modulated by extracellular matrix proteins and the blockade of b#-integrin signaling. These culture models allow to evaluate inhibitory or stimulative effects on single candidate molecules in regulatory pathways that are involved in nuclear b-catenin accumulation and growth behaviour.
Our goal is to investigate basic mechanisms of malignant tumor progression to discover and validate new diagno¬stic markers for tumor prediction and new therapeutic targets for interruption of CRC progression. This will be achieved by profiling the transcriptional regulation and the non-linear dynamism of invasion in primary tumors and their metas¬tases.
A remarkable finding of our work is that the expression of a number of genes is different between non-metastatic and metastatic primary colorectal carcinomas. These results demonstrate that the expression of critical genes is altered in the majority of cells in metastatic primary tumors, similar to metastases. Our data are in agreement with recent findings of other groups, that metastatic cells are not rare variants of primary tumors (Ramaswamy et al., 2003; van 't Veer et al., 2002). We conclude that our signature genes link growth with the ability to metastasize, and thus favour the spread of metastatic variants within the primary tumor (see Bernards and Weinberg, 2002).
We also could effectively assign expression profiles of metastases to the metastatic class, even in cases where primary tumors were not analyzed. Our findings thus demonstrate that the analysis of gene signatures of human colorectal cancers is useful in the clinic to predict the occurance of metastasis and is to the benefit of patients.
Lit.: 1. Kirchner T et al. Patterning and nuclear beta-catenin expression in the colonic adenoma- carcinoma sequence : analogies with embryonic gastrulation. Am J Pathol. 2000;157(4):1113-21. 2. Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nature Reviews Cancer. 2002;2(442-54. 3. Kinzler KW et al. Lessons from hereditary colorectal cancer. Cell. 1996;87(2):159-70. 4. Brabletz T et al. Variable beta-catenin expression in colorectal cancer indicates a tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001 August 28, 2001;98(18):10356-61. 5. Barker N et al. Tumor environ¬ment: a potent driving force in colorectal cancer? Trends Mol Med. 2001;7(12):535-7. 6. Brabletz T et al. Invasion and Metastasis in Colorectal Cancer: Epithelial-Mesenchymal Transition, Mesenchymal-Epithelial Transition, Stem Cells and b-Catenin. Cells Tissues Organs. 2005; 179(1-2):56-65. 7. Brabletz T et al. Migrating cancer stem cells - an integrated concept of malignant tumour pro¬gression. Nature Reviews Cancer. 2005 (accepted). 8. Brabletz T et al. beta-Catenin Regulates the Expression of the Matrix Metallo¬proteinase-7 in Human Colorectal Cancer. Am J Pathol. 1999 October 1, 1999;155(4):1033-38. 9. Hlubek F et al. Expression of the invasion factor laminin g2 in colorectal carcinomas is regulated by b-catenin. Cancer Res. 2001; 61(8089-93. 10. Jung A et al. The Invasion Front of Human Colorectal Adenocarcinomas Shows Co- Localization of Nuclear beta-Catenin, Cyclin D(1), and p16(INK4A) and Is a Region of Low Proliferation. Am J Pathol. 2001;159(5): 1613-7. 11. Hlubek F et al. beta-Catenin activates a coordinated expression of the proinvasive factors laminin-5 gamma2 chain and MT1-MMP in colorectal carcinomas. Int J Cancer. 2004 Jan 10;108(2):321-6. 12. Hiendlmeyer E et al. Beta-catenin up-regulates the expression of the urokinase plasminogen activator in human colorectal tumors. Cancer Res. 2004 Feb 15;64(4):1209-14. 13. Beiter K et al. beta-Catenin regulates the expression of tenascin-C in human colorectal tumors. 2005 2005/08/01/online.


