
Introduction
Colorectal carcinomas result from accumulating mutations over many years. A subgroup of colon cancers will metastasize almost immediately, whereas other tumors with similar histopathological characteristics have a long clinical course or never relapse. To date, colon tumors with different outcome cannot be distinguished by means of clinical or histopathological examination. For instance, patients who will not profit from chemotherary or radiation still receive standardized, aggressive therapy. This results in an unjustified loss in quality of life and also in a cost-inefficient treatment. Much has been learned in recent years about mutations that control initiation and progression of colorectal cancer. Less is known about molecular events that are crucial in metastasis formation, which is the fatal step in the disease.
Initiation of colon cancer is controlled by mutations of genes of the Wnt/ß-catenin pathway, such as of adenomatous polyposis coli (APC), Axin or ß-catenin which regulate ß-catenin’s stability (reviewed in Bienz and Clevers, 2000; Polakis, 2000). Progression of colon cancer is controlled by mutations of genes of the ras and the TGF-ß pathways, such as k-ras, the TGF-ß receptor II, and Smad’s, as well as by mutations of p53. Metastasis of colon cancer has not been linked to particular signalling pathways, however, the phosphatase PRL-3 is upregulated in metastases (Saha et al., 2001). The Wnt/ß-catenin pathway is also involved in progression of colon cancer, since ß-catenin becomes nuclear at the invasive fronts of primary tumors late in tumorigenesis (Brabletz et al., 1998).
In a series of recent studies, microarray profiles of metastases and primary tumors have been compared (van 't Veer et al., 2002; Ramaswamy et al., 2003). These array studies demonstrate that gene expression signatures could be identified that characterize both, metastases and primary tumors with high metastatic potential, but not non-metastatic primary tumors. These data thus imply that metastatic variants in the primary tumors are generally not rare, but represent a considerable fraction of the primary tumor. In accordance, prediction of breast cancer metastasis was possible by examination of particular gene signatures in the primary tumors (van’t Veer et al., 2002).
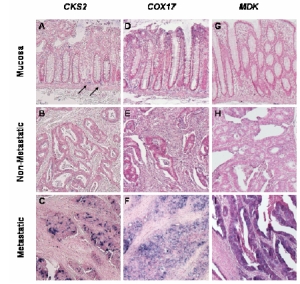 | |
| Fig 1: Expression patterns of selected genes in normal colon mucosa and tumor sections as determined by in situ hybridization. | |
Results
The Department of Surgical Oncology of the Robert Roessle Cancer Clinic in Berlin-Buch has established a comprehensive tumor tissue bank of primary colon cancers and metastases, which also lists histopathological and follow-up data of patients. From this bank, we selected around 100 locally advanced metastatic and non-metastatic primary tumors, as well as metastases to lymph nodes, liver, and lung. Normal colon tissue samples were also included. Tissue of epithelial origin was isolated by laser-capture microdissection, RNA was purified, and examined by Affymetrix expression profiling (10,000 genes were tested).
Thus, a gene signature could be identified that distinguished metastatic primary from non-metastatic primary tumors. Linear regression analysis demonstrated that non-metastatic (nPT) and metastatic primary tumors (mPT) could indeed be correctly classified. Many of the signature genes code for signalling molecules or transcription factors, consistent with the role of these genes in the regualtion of complex cell behavior. After validation by in situ hybridization (Fig.1), we further analyzed selected signature genes, and could (i) demonstrate differential expression in an independent group of primary metastatic and non-metastatic colorectal tumors, (ii) correlate signature gene expression to patient survival, (iii) link individual genes to signalling pathways that are important for tumor progression, and (iiii) demonstrate the importance of these signalling pathways for tumor metastasis. These data thus demonstrate that specific gene expression is modulated in a significant fraction of colorectal cancers with high metastatic potential, and that this expression is of prognostic value for patients survival.
Outlook
A remarkable finding of our work is that the expression of a number of genes is different between non-metastatic and metastatic primary colorectal carcinomas. These results demonstrate that the expression of critical genes is altered in the majority of cells in metastatic primary tumors, similar to metastases. Our data are in agreement with recent findings of other groups, that metastatic cells are not rare variants of primary tumors (Ramaswamy et al., 2003; van 't Veer et al., 2002). We conclude that our signature genes link growth with the ability to metastasize, and thus favour the spread of metastatic variants within the primary tumor (see Bernards and Weinberg, 2002).
We also could effectively assign expression profiles of metastases to the metastatic class, even in cases where primary tumors were not analyzed. Our findings thus demonstrate that the analysis of gene signatures of human colorectal cancers is useful in the clinic to predict the occurance of metastasis and is to the benefit of patients.
Lit.: 1. Bernards, R. and Weinberg R. (2002). A progression puzzle. Nature 418, 823. 2. Bienz, M., and Clevers, H. (2000). Linking colorectal cancer to Wnt signaling. Cell 103, 311-320. 3. Brabletz, T., Jung, A., Hermann, K., Gunther, K., Hohenberger, W., and Kirchner, T. (1998). Nuclear overexpression of the oncoprotein beta-catenin in colorectal cancer is localized predominantly at the invasion front. Pathol Res Pract 194, 701-704. 4. Polakis, P. (2000). Wnt signaling and cancer. Genes Dev 14, 1837-1851. 5. Ramaswamy, S., Ross, K. N., Lander, E. S., and Golub, T. R. (2003). A molecular signature of metastasis in primary solid tumors. Nat Genet 33, 49-54. 6. Saha, S., Bardelli, A., Buckhaults, P., Velculescu, V. E., Rago, C., St Croix, B., Romans, K. E., Choti, M. A., Lengauer, C., Kinzler, K. W., and Vogelstein, B. (2001). A phosphatase associated with metastasis of colorectal cancer. Science 294, 1343-1346. 7. van 't Veer, L. J., Dai, H., van de Vijver, M. J., He, Y. D., Hart, A. A., Mao, M., Peterse, H. L., van der Kooy, K., Marton, M. J., Witteveen, A. T., et al. (2002). Gene expression profiling predicts clinical outcome of breast cancer. Nature 415, 530-536.


