
Introduction
Stem cells have been defined by two characteristics that distinguishes them from other types of cells: 1) they are unspecialized cells that renew for a lifetime by cell division to maintain the stem cell pool and 2), they can differentiate to cells with special functions under certain physiologic or experimental conditions. To fulfill this dual function, they must undergo symmetric and asymmetric divisions during development [1].
While some types of stem cells are already used for clinical therapy, recent investigations raised high hopes for a broad spectrum of applications in cell-based therapies for regenerative or reparative medicine. It has been hypothesized that stem cells may at some point in the future become the basis for treatment of diseases such as Parkinson’s disease, diabetes and heart disease. Furthermore, the regenerative potential of a living organism is linked to the ability of its stem cells to replace the corresponding damaged tissue. In this context the complex process of aging involving every cell and organ in the body could also be interpreted as signs of aging at the level of somatic stem cells. A living organism is as old as its stem cells and hence they seem to play a central role in aging [2]. Stem cells are one of the most fascinating areas of biology today. But like many expanding fields of scientific inquiry research on stem cells raises scientific questions as rapidly as it generates new discoveries [3]. A central question in stem cell research remains to elucidate their essential properties and what makes them different from specialized cell types on a molecular level.
Adult stem cells or progenitor cells maintain and repair the tissue in which they are found in living organisms throughout their life. These somatic stem cells have recently generated a great deal of excitement. Certain kinds of adult stem cells seem to have the ability to form specialized cell types of other tissues or other germinal layers, which is known as transdifferentiation or plasticity. Although still heavily debated, this pluripotent potential has been suggested by a number of experiments: For example hematopoietic stem cells (HSC) may differentiate into the three major types of brain cells (neurons, oligodendrocytes and astrocytes), skeletal muscle cells, cardiac muscle cells and liver cells; and mesenchymal stem cells (MSC) may differentiate into cardia muscle cells and skeletal muscle. However, some of the initial reports could not be reproduced. Quality control in the laboratory represents a conditio sine qua non for these cells to become the basis of therapies.
Regulation of stem cell function depends largely on their interaction with a highly specialized microenvironment, the so called “stem cell niche”. Self-renewal and differentiation of adult stem cells need to be tightly regulated according to the physiologic needs of the organism. This has been proven for a variety of different types of stem cells in different animal models. In the hematopoietic system osteoblasts lining the bone surface function as a major constituent of the hematopoietic stem cell niche. The niche involves signals for localization, expansion and constraint of stem cells and therefore defining the parameters that control these phenomena offers the potential for manipulating them to achieve therapeutic effects. Supportive feeder layer cells provide an in vitro model system for interaction with the stem cell niche. Co-cultivation with feeder layer cells maintains the self-renewing ability of embryonic stem cell lines in vitro. In analogy stromal layers have been demonstrated to play a vital role for growth and development of hematopoietc progenitor cells in vitro. Direct contact and communication between stem cells and cellular determinants of the microenvironment has been shown to play an essential role in this process [4]. Although numerous studies have demonstrated the supportive role of stroma feeder layers for maintenance of multi-potency of HPC in vitro little is known on the precise cellular and molecular mechanisms of this interaction. The interplay of stem cells and their niche forms a functional unit that is of interest for stem cell biology.
Our current research focuses on 1) determining molecular characteristics of HSC and MSC; 2) analysis of cell-cell interaction of HSC and their niche and 3) investigation of the molecular sequele
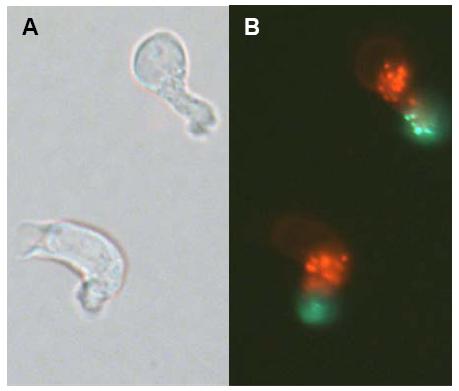
Fig 1: Hematopoietic stem cells are enriched in the CD34+/CD38- cell fraction and can be further enriched in the slow dividing fraction (SDF). As demonstrated in this figure these cells demonstrate a heterogeneous morphology with strong podia formation and CD133 is located at the tip of the uropod at the trailing edge (A: phase contrast; B: fluorescence [red: residual PKH26 staining; green: CD133]).
Global gene expression analysis might provide a new dimension for understanding of the unique molecular features of stem cells. Various studies have determined genome wide gene expression profiles of hematopoietic stem cells (HSC) but these efforts are limited by the heterogeneity of populations using the available methods for enrichment. We have provided evidence that divisional kinetics could be exploited to separate progenitor cells into two fractions: a slow dividing fraction (SDF) that is predominantly associated with primitive function and self-renewal, and a fast dividing fraction (FDF) that divides symmetrically and predominantly proceeds to differentiation [5].
In continuation with this line of research we have analyzed the global gene expression profiles of these two populations [6]. Gene expression analysis was performed with a novel “Human Transcriptome Microarray” that represents 51,145 UnigeneSet-RZPD3 cDNA clones. To obtain additional information on primitive versus committed progenitor cells, gene expression profile of CD34+/CD38- cells, which are associated with more primitive function was compared with that of the CD34+/CD38+ cells that are committed to differentiation. In the CD34+/CD38- fraction 96 sequences were found to be at least two-fold over-expressed and 119 were suppressed in comparison to CD34+/CD38+ cells. In contrast in the SDF versus the FDF a larger proportion of genes was differentially expressed (942 sequences up-regulated and 794 down-regulated). Among the genes showing the highest expression levels in the SDF were the following: CD133, ERG, cyclin g2, MDR1, osteopontin (SPP1), C1QR1, IFI16, JAK3, FZD6 and HOXA9, a pattern compatible with their primitive function and self-renewal capacity. In comparison to three other published microarray studies we identified several genes that were up-regulated in different fractions of murine and human HSC including: HOXA9, JAK3, FZD6, MDR1 and RBPMS. Furthermore we observed significantly more podia-formation in the SDF and CD133 is located on the tip of these podia (Fig 1). In addition we have analyzed whether retroviral integration sites in repopulating hematopoietic cells correlate with genes up-regulated in HSC. Thereby retroviral integrations could mark genes that are initially expressed in the small subset of repopulating cells. Statistical analysis showed that retrovirial integration occurred preferentially in genes that were higher expressed in cell CD34+/CD38- cells (as compared to CD34+/CD38+ cells) and in the SDF (as compared to the FDF). These results demonstrate the association of gene expression and retrovirally targeted genes in primitive repopulating hematopoietic cells. A collaborative RISC score database (CRSD) of the gene therapy safety group (www.gtsg.org) has been set up to collect further information about retroviral integration sites. Thus, integrated analysis of retroviral integration sites can assist gene expression analysis of repopulating HSC and their daughter cells [7].
Interaction with the stem cell niche
Cell-cell contact between stem cells and cellular determinants of the microenvironment plays an essential role in the regulation of self-renewal and differentiation. The stromal cell line AFT024 derived from murine fetal liver has been shown to support maintenance of primitive human hematopoietic progenitor cells (HPC) in vitro. We have studied the interaction between HPC and AFT024 and the impact of co-cultivation on the behavior and gene expression of HPC. Time lapse microscopy revealed that approximately 30% of the CD34+/CD38- cells adhered to the cellular niche through an the pseudopodium at the trailing edge (uropod). CD44 and CD34 were co-localized at the site of contact. Gene expression profiles of CD34+/CD38- cells were then compared upon co-cultivation either with or without AFT024. After cultivation for 16h, 20h, 48h or 72h the HPC were separated form the feeder layer cells by a second FAC-Sort. Among the genes with the highest up-regulation in contact with AFT024 were several genes involved in cell adhesion, proliferation and DNA-modification. Our studies support the hypothesis that intimate contact and adhesive interaction of HPC with their niche profoundly influenced their proliferative potential and their differentiation program [8].
What is a Mesenchymal Stem Cell?
Mesenchymal stem cells (MSC) represent another archetype of multipotent somatic stem cells that give rise to a variety of cell types including osteocytes, chondrocytes, adipocytes and other kinds of connective tissue cells such as those in tendons. However, molecular markers that define MSC are so far elusive and under this aspect the above mentioned question is not easy to answer. Various preparative protocols have been proposed for acquisition and cultivation of MSC. Whereas surface antigen markers have failed to precisely define this population, microarray analysis might provide a better tool for characterization of MSC.
We have analyzed the molecular signatures of human MSC isolated from bone-marrow under two different culture conditions (BM-MSC-M1 and BM-MSC-M2), from adipose tissue (AT-MSC-M1) and from umbilical cord blood (CB-MSC-M3) in comparison to non-multipotent human fibroblasts (HS68 and NHDF). Osteogenic and adipogenic differentiation potentials could be demonstrated in all MSC preparations but not in fibroblasts. No phenotypic differences were observed by flow cytometry using a panel of 22 surface antigen markers. Global gene expression profiles were then compared using our Human Genome cDNA Microarray. Twenty-five genes were overlapping more than two-fold up-regulated in all MSC preparations and these included fibronectin, ECM2, glypican-4, ID1, NF1B, HOXA5 and HOXB6 whereas several inhibitors of the Wnt-pathway (DKK1, DKK3, SFRP1) were higher expressed in fibroblasts. Gene expression profiles indicated that under standardized conditions similar cell populations could be reproducibly isolated whereas there were significant differences between different cell preparation procedures and MSC from different tissues. Our comparative approach provides foundation for a reliable quality control using genotypic analysis for clinical applications.
Outlook
Gene and protein expression analysis of stem cell preparations might provide another dimension for defining specific adult stem cell types. The correlation between protein and gene expression profiles of specific subsets of stem cells and their ultimate differentiation potentials will provide relevant information on the genetic determinants of specific pathways of differentiation. In addition functional studies will be required to verify the significance of identified genes. Knowledge gained in this line of research will provide foundation for a reliable quality control of stem cell preparations for specific purposes.
Lit.: 1. Ho AD. Kinetics and symmetry of divisions of hematopoietic stem cells. Exp.Hematol. 2005;33:1-8. 2. Ho AD, Wagner W, Mahlknecht U. Stem cells and ageing. EMBO reports. 2005;in press. 3. Ho AD, Punzel M. Hematopoietic stem cells: can old cells learn new tricks? J.Leukoc.Biol. 2003;73:547-555. 4. Punzel M, Liu D, Zhang T et al. The symmetry of initial divisions of human hematopoietic progenitors is altered only by the cellular microenvironment. Exp.Hematol. 2003;31:339-347. 5. Huang S, Law P, Francis K et al. Symmetry of initial cell divisions among primitive hematopoietic progenitors is independent of ontogenic age and regulatory molecules. Blood 1999;94:2595-2604. 6. Wagner W, Ansorge A, Wirkner U et al. Molecular evidence for stem cell function of the slow-dividing fraction among human hematopoietic progenitor cells by genome-wide analysis. Blood 2004;104:675-686. 7. Wagner W, Laufs S, Blake J et al. Retroviral Integration Sites Correlate with Expressed Genes in Hematopoietic Stem Cells. Stem Cells 2005. 8. Wagner W, Saffrich R, Wirkner U et al. Hematopoietic Progenitor Cells and Cellular Microenvironment - Behavioral and Molecular Changes upon Interaction. Stem Cells 2005.


