
Introduction
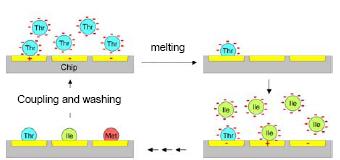
Fig 1: Deposition of amino acid particles on the chip using electrostatic forces. Charge patterns on the chip surface are generated and configured by the microchip itself.
The objective of this research project is the development of a method for generating peptide arrays of high complexity. These arrays should allow for the fast and comprehensive translation of the large number of amino acid sequences stored in databases into real protein fragments on a support. As first applications, we aim at representing the proteomes of Borrelia burgdorferi and Mycobacter tuberculosis as a library of overlapping peptides on such an array. After incubation with sera of patients with lyme disease or tuberculosis, we expect to observe patient-dependant interaction patterns of serum antibodies with the individual peptides on the chip, which correlate both with differences in the disease processes and with the severity of the symptoms. In the long run, the employment of such arrays will enable the differential diagnosis of these diseases manifested in individual patients.
Although first commercial products are already available, peptide and protein microarrays are not at hand in the extent desired by the medical and biological research yet. Therefore, a new method to parallelize the combinatorial synthesis of peptide libraries is being developed in this joint project of KIP (1) and DKFZ (2).
In order to perform high-density combinatorial chemistry, a method to deposit the reagent on a suitable support with high spatial precision is needed. One common technology today is the use of micro-pipettes, but the limits of miniaturization for this technology are already set: On one hand, it is necessary to accurately align the pipettes to the support in order to maintain the synthesis addresses repeatedly. On the other hand, a certain distance between spots must be kept in order to prevent the solvent drops of neighbouring spots from merging. Alternative methods like various printing technologies suffer from at least one of these restrictions.
However, a procedure free from positioning inaccuracies can be realized by utilizing an “active” support containing actuators coinciding with the synthesis addresses. These actuators can reproducibly determine the spots on which a reagent is deposited in each reaction step. Such a process would be self-aligned, bypassing the need for external precision alignment. A prerequisite of such an active support is the arbitrary configuration of individual spots to enable or disable reagent uptake in different reaction steps.
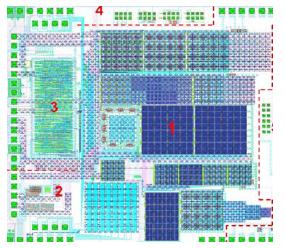
Fig 2: Layout of the peptide chip. 1) Sector with different test pixels. 2) Generation of bias tensions. 3) I2C-Interface 4) High voltage transistors for testing purposes.
Repeating addressing and synthesis enables us to build up a peptide array with a large number of peptides in parallel, whereby the peptide length is defined by the number of combinatorial cycles.
Project Status
Chip design Requirements for the CMOS chip (complementary metal oxides semiconductor) were determined in preliminary tests, using simple electrode structures. Furthermore, the different forces affecting the deposition of conventional color toner particles were assayed. In particular, the electrostatic force is crucial for the deposition selectivity of the particles. The contrast between different electrodes depends directly on differences of the electrostatic potential. Therefore, different electronic circuits were evaluated with special CAD (computer aided design) software and, as a result, a corresponding integrated circuit with adjustable voltages up to 100V was designed. The chip, which has already been manufactured, contains pixels which combine high- and low-voltage circuits on a small area. Different kinds of pixel electrodes are implemented in the chip and organized in sectors as shown in Fig. 2. They can be used to characterize the quality of particle transfer as a function of the shape, structure, and size of the pixels, and also as a function of the voltage applied. The chip is controlled and configured via a digital control interface using the I2C protocol. This interface allows to program arbitrary coating patterns for the different pixels. The functionality of large parts of the chip, especially with respect to particle transfer, has already been proven in first tests (see below). Protein-peptide interactions will be assayed by labeling with optical markers. Therefore, some sectors on this chip already contain pixels with integrated photodiodes for this purpose. Additional test structures were implemented for a thorough characterization of all kind of photodiodes and for measuring the high voltage transistors individually.
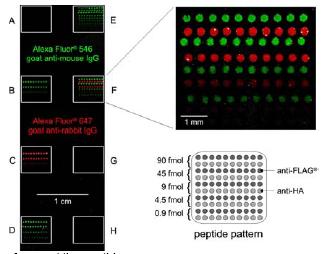
Fig 3: Chip surface coated with two different laser printer toners from an aerosol.
So far, 12 different sorts of amino acid particles have been developed. Besides the activated amino acids, these particles contain a “solid solvent” diphenylformamide for the chemical reaction, as well as additives for the triboelectric charging of the particles. Among these additives are polymer resins and metalorganic complexes, so called "charge control agents" and "charge stabilizer", depending on their respective function. The particles were optimized regarding their physical and chemical characteristics for combinatorial synthesis (coupling yield, interaction with additives, cleaning) and high resolution addressing on the chip (charge-to-mass ratio, triboelectric charging, size, size distribution, agglomeration etc.). A synthesis of the Flag epitope using amino acid particles resulted in repetitive yields of about 94%, which corresponds to the results obtained with classical Merrifield synthesis. The particles were produced with an air jet mill. The size distribution of the particles yielded an average diameter of less than 10µm, which is in line with commercial color toners. The charge-to-mass ratio of the monomer particles, which determines the transfer characteristics of the particles from an aerosol on the chip surface, is variable over a broad range. This ratio was adjusted to a reference value, using conventional OKI color toner (OKI C7000-series) as reference.
Particle transfer
Transfer experiments with the chip and commercial color toners demonstrated the particle deposition on chip surface with good contrast and high resolution (Fig. 3). In particular, it was shown that the minimum coating voltage is 20V and that the contrast increases with higher voltages. Furthermore, amino acid particles were also deposited with minimal contaminations on pixel electrodes ranging from 100µm to 43µm pitch. For more complex patterns, improved coating quality was achieved by separating the pixels using a narrow deflecting grid electrode set at a fixed voltage.
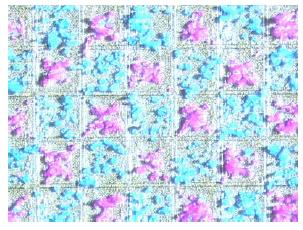
Fig 4: Protein-resistant glass slide with spotted peptides representing the FLAG® and the HA epitope. The slide was stained with the corresponding antibodies in the absence of blocking agents.
The detection limit of a system for the characterization of biochemical interactions crucially depends on the achievable signal-to-noise ratio. For this reason, the suppression of non-specific signals is very important. Nowadays, protein mixtures such as milk powder are usually used in order to suppress non-specific binding of probes to the support material. However, even after several washing steps, about 15% of the signal is still attributed to non-specific interactions of proteins with the support. This can be avoided using water-soluble graft-polymerised poly(ethylenglykol)-methacrylate [PEGMA]: These films generally protect from non-specific protein adsorption, and furthermore allow for adjusting the surface density of functional groups. The OH-terminated PEGMA films can be NH2-terminated by coupling of Fmoc-ß-alanin to enable a combinatorial peptide synthesis according to Merrifield. Thus, the PEGMA film serves as a linker between the chip A detailed analysis of the surface with IR and XP spectroscopy as well as ellipsometry confirmed protein resistance of the substrate even to sticky proteins like fibrinogen. In a first experiment, pre-synthesised peptides (Flag and HA epitopes) were spotted onto a modified glass surface (Fig. 4). Treatment with specific antibodies resulted in a specific peptide staining with negligible background even in the absence of additional blocking agents.
Outlook
The results obtained from the experiments with the current chip will determine the design of the next generation of application-ready peptide chips. Flag and HA epitopes will be produced on these chips using the available amino acid particles, followed by detection with the corresponding antibodies. After the gerenation of all sorts of amino acid particles, proteins of Borrelia burgdorferi will be manufactured as a set of overlapping peptides. In order to simplify and standardize the synthesis, a semi-automatic device for particle deposition and synthesis cycles will be constructed. The peptide arrays of Borrelia burgdorferi will be incubated with sera from infected patients, allowing the characterization of antibody response. Detection of binding events will be achieved using the integrated photodiodes. A software for analyzing the interaction patterns is under way.
Lit.: 1. Kai König, Gloria Torralba, Ulrich Trunk, Volker Lindenstruth, Kirchhoff Institute of Physics (KIP), Heidelberg. 2. Volker Stadler, Alexander Nesterov, Mario Beyer, Thomas Felgenhauer, Ines Block, Simon Fernandez, Dorothea Boy, Thorsten Kühlwein, Klaus Leibe, F. Ralf Bischoff, Frank Breitling, German Cancer Research Center (DKFZ) Heidelberg.


