
Introduction
A study by the World Health Organization has found that diseases of the central nervous system (CNS) are affecting a significant portion of the population and are amongst the leading causes of death in Western societies (1,2). Although great advances have been made in our understanding of the pathophysiology of psychiatric and neurological diseases, significant gaps remain in our knowledge about their ultimate causes. A major problem in the area of affective diseases is the fact that current diagnosis is mainly based on categorizing the signs and symptoms of the syndrome, which limits the ability to reliably identify biological findings and develop specific treatments. Unlike other disorders like diabetes and heart disease where biological markers are at hand that allow the physician to come up with a reliable diagnosis there are currently no such markers available for affective diseases. Here the only means for diagnosis is the Diagnostic and Statistical Manual of Mental Disorders (DSM) (3). This manual systematically identifies different mental illnesses according to a list of symptoms but does in no way address the underlying cause of the disease. A major goal in the area of mental disorders is therefore the identification of markers that can categorize subsets of subjects in a consistent manner. This will allow a more precise definition and categorization of affective disorders and in turn facilitate investigations of the pathogenesis of the diseases and enhance our ability for treatment. Autoantibodies against CNS proteins in serum and cerebrospinal fluid (CSF) of patients with affective disorders have been described in several studies (4,5). In one such study it was found that an increased immunoglobulin (Ig) G titer was mainly present in patient’s CSF and serum during a state of depression (5). Furthermore, besides an immune dysfunction there is evidence for an impaired blood-brain barrier in affective disorder patients, which increases the susceptibility for an autoimmune response (4). An excessive IgG transmission from blood into CSF and/or an increased synthesis of IgG in CSF, in combination with elevated monocyte and T lymphocyte activities, may ultimately result in an unusually high antibody surveillance in the CNS. The uncommon exposure to an increased immune response in turn might lead to an increased risk of mal-recognition of certain endogenous proteins that are present and share resemblance to exogenous antigens.
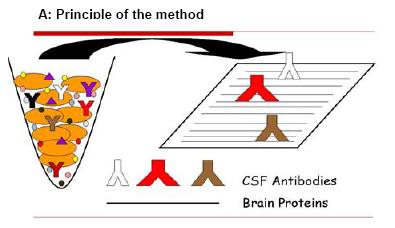
Fig 1: CSF Western blot analysis. Human brain proteins immobilized on a membrane are probed with CSF antibodies (A). If the CSF contains a brain-specific antibody it will bind to its antigen on the membrane and generate a signal that can be detected by an X-ray film (B).
The focus of my research laboratory is the identification of disease markers for affective disorders. To this end we have concentrated our efforts on the analysis of the protein constituents in CSF with modern proteomic technologies (8-10). These efforts will continued to be pursued on several levels. At the same time, however, we have begun to establish alternative strategies for the identification of novel disease markers for affective disorders. In this regard we have begun to exploit the great specificity of the body’s immune system. Specifically, we are analyzing the antibody pool that is present in CSF. The basis of this Exploratory Project that is supported by NGFN-2 stands on the hypothesis that several factors may trigger an autoimmune response within the CNS especially in individuals with affective disorders that are propositioned to immune system dysfunction and impaired blood-brain barriers. We hypothesize that the presence of markers for affective diseases is reflected by the appearance of autoantibodies in CSF. These autoantibodies can be used for the identification of their respective autoantigen counterparts that consequently represent markers of disease. The goal of the Exploratory Project is to identify and then validate novel candidate markers for brain diseases.
Project Status
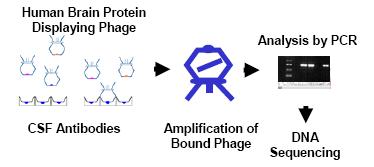
Fig 2: Biomarker identification with CSF antibodies by phage display screen. Antibodies derived from CSF are immobilized on magnetic beads and subjected to panning with a human brain protein-expressing phage display library. After several rounds of panning the bound phage is amplified, analyzed by PCR and the insert DNA sequenced for autoantigen identification.
In preliminary experiments we have established a Western blot immunoassay for the evaluation of CSF samples (Fig. 1). In order to detect brain-specific autoantibodies in CSF we subjected a human brain protein extract to SDS gel electrophoresis. The size separated brain proteins were subsequently transferred to a membrane and then probed with different CSF samples.
Fig. 1 outlines the principle of the method (Fig. 1A) and shows our preliminary results of such a CSF Western blot (Fig. 1B). As is evident from the image there are discrete protein bands that represent brain proteins specifically recognized by autoantibodies that are present in the CSF.
Although the CSF Western blot method demonstrates that specific autoantibodies are present in CSF and can be detected using biochemical techniques, the identity of the autoantigens remains obscure. The realization that the identification of brain autoantigens is limited by the CSF sample that is available prompted us to explore alternative experimental strategies. The extreme sensitivity of the phage display approach (11) led us to employ as the key technology of our Exploratory Project. We therefore reasoned that phage display would greatly improve our chances of discovering autoantigens that are expressed at low levels in the brain. In this case brain peptides and proteins are displayed on the surface of a phage particle and provide a direct link between phenotype and genotype. Every displayed protein has an addressable tag via the DNA encoding that protein. Phage with the brain protein displayed can be isolated from large libraries by a series of repeated selection cycles (panning) on the immobilized antibodies, each of which involves binding, washing, elution and amplification.
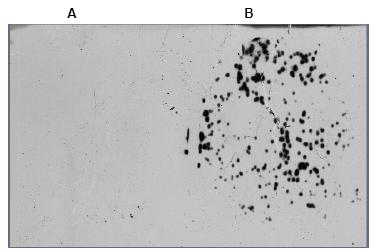
Fig 3: After phage display enrichment with anti-synuclein-specific antibody plaque lifts of the bound phage were performed. A: filter from control (no antibody) panning; B: filter from anti-synuclein antibody panning.
Outlook
Taking all the necessary precautions allows us now to use the CSF antibody fraction from patients with affective disorders for biopanning. We are in the process of screening CSF of patients with unipolar depression (UPD), bipolar disorder (BPD) and schizophrenia as well as matched controls. In addition to being valuable marker proteins for affective disorders the identified autoantigens will for the first time allow the localization of pathological lesions for these diseases. Several methods will be employed to narrow down the site of expression of the relevant autoantigens. For the most precise localization of the autoantigen marker, we plan to subject brain tissue slices to in situ hybridization. The identification and tissue localization of autoantigens specific for BPD and UPD will lead to further studies designed to investigate the possible function of the proteins in the pathogenesis of these diseases.
Follow up studies that are beyond the scope of the current application will include gene silencing via RNA interference, transfection assays and expression analysis in in-vitro and in-vivo models. Ultimately, these studies will aim at the development of therapeutic compounds and diagnostic biomarkers for affective disorders that are characterized by an unprecedented specificity. These could include antigen-specific tolerizing therapies and microarray-based autoantibody profiling (12) with the potential
Lit.: 1. Holsboer, F. (2001). Antidepressant drug discovery in the postgenomic era. World J. Biol. Psychiatry 2, 165-177. 2. Blazer, D.G. (2000). Mood disorders: epidemiology. In: Comprehensive textbook of psychiatry. Pp 1298-1308. 3. Diagnostic and Statistical Manual of Mental Disorders, 4th Ed. 4. Wang, X.-F., Wang, D., Zhu, W., Delrahim, K.K:, Dolnak, D., Rapaport M.H. (2003). Studies characterizing 60 kDa autoantibodies in subjects with schizophrenia. Biol. Psychiatry 53, 361-375. 5. Hornig, M., Amsterdam, J.D., Kamoun, M., Goodman, D.B.P. (1999). Autoantibody disturbances in affective disorders: a function of age and gender? J. Affective Disorder 55, 29-37. 6. Nowatzke, W.L., Parvin, C.A., Scott, M.G., Hock, K., Cole, T.G. (2001). Correction of positive bias of the Roche Tina-quant II hemoglobin A1c (HbA1c) assay at low HbA1c percentages. Clin. Chem. 47, 976-978. 7. Boyle, D.L., Rosengren, S., Bugbee, W., Kavanaugh, A., Firestein, G.S. (2003). Quantitative biomarker analysis of synovial gene expression by real-time PCR. Arthritis Res. Ther. 5, R352-R360. 8. Maccarrone, G., Milfay, D., Birg, I., Rosenhagen, M., Grimm, R., Bailey, J., Zolotarjova, N., Turck, C.W. (2004). Mining the human CSF proteome by immunodepletion and shotgun mass spectrometry. Electrophoresis 25, 2402-2412. 9. Maccarrone, G., Birg, I., Malisch, E., Rosenhagen, M.C., Ditzen, C., Chakel, J.A., Mandel, F., Reimann, A., Doertbudak, C.-C., Haegler, K., Holsboer, F. and Turck, C.W. (2004). In-depth analysis of the human CSF proteome using protein prefractionation. Clin. Proteomics J., 1, 333-364. 10. Turck, C.W. Brain disorder biomarker discovery. (2005). Laborwelt, 6, 4-10. 11. Danner, S., Belasco, J.G. (2001). T7 phage display: a novel genetic selection system for cloning RNA-binding proteins from cDNA libraries. Proc. Natl. Acad. Sci. USA 98, 12954-12959. 12. Robinson, W.H., Steinmann, L., Utz, P.J. (2002). Protein and peptide array analysis of autoimmune disease. Biotechniques Dec. Suppl., 66-68.


