Introduction
Atopy is defined as familial tendency to develop characteristic IgE-mediated allergic diseases on the basis of skin and mucuous membrane hyperreactivity (1). According to epidemiological studies, the number of human individuals suffering from atopic diseases of the skin and the airways (atopic dermatitis, allergic asthma, allergic rhinoconjunctivitis) has increased over the last decades (2). The human genetic background together with environmental factors is thought to be responsible for the almost epidemic increase of IgE-mediated allergic diseases in recent years (2). Major recent advances in the field of molecular genetics, especially the sequencing of the human and the mouse genomes, provide great opportunities for a better understanding of the basic mechanisms underlying IgE-mediated allergic diseases. Family and twin studies have already clearly indicated that the genetic contribution to atopic diseases is more than substantial (3). However, the precise function and linkage to allergic disease has only been provided for a limited number of individual genes. Understanding the complex genetic program that leads to the precipitation of such phenotypically distinct diseases in atopy will be essential for the development of new diagnostic and therapeutic approaches in IgE-mediated allergy. In this respect, the development of phenotypically and genotypically defined animal models will be an important step.
The primary goal of the Allergy Screen at the German Mouse Clinic (GMC) is to identify defined mouse mutant lines, which can serve as representative models for human allergic diseases (atopic dermatitis, allergic asthma, allergic rhinoconjunctivitis, food allergy, anaphylaxis). To achieve this primary goal, we have established a high-throughput screening approach on the basis of total plasma IgE levels. Allergy prone mutants identified in this way undergo, in collaboration with the mouse providers, an in-depth secondary and tertiary screening based on allergic sensitization and allergen re-exposure protocols.
Results
Screen status
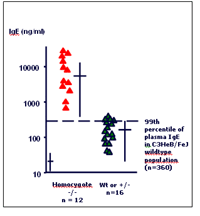
We have previously established total plasma IgE levels as a powerful parameter to detect allergy prone mutants in a systematic screening effort such as the ENU mouse mutagenesis program (4). In the ENU program, the systematic screen for dominant and recessive mutations affecting plasma IgE levels has led to the identification of 17 mutant mouse lines (MML) with abnormal plasma IgE levels. One of the IgE MML has been mapped to a 2.1 cM region on murine chromosome 1 and the genetic mutation of interest turned out to be a point mutation in the T lymphocyte signal transducing protein ZAP70 (Jakob et al., publication in preparation; Figure 1). Since the start of the GMC we have adopted total plasma IgE as our primary screening parameter for the analysis of peripheral murine blood samples to detect allergic phenotypes. To facilitate large-scale automated screening for relevant allergic phenotypes, a high-throughput technology was established at the GMC together with the Immunology Screen (PMM-S31T11; D. Busch, TU Munich). Standard operation procedures (SOP) were precisely defined for all working steps of the total plasma IgE assay, allowing very sensitive and highly reproducible measurements of this crucial parameter. For validation purposes of our SOPs, we analyzed a variety of commonly used mouse inbred strains (BALB/c, C57BL/6, C3HeB/FeJ) with the primary screening protocol. The dataset provides reference values for total IgE in murine plasma, thus representing an invaluable information for future studies on mutant mice entering the GMC. The comparison of our data revealed that we are able to precisely reproduce known strain and sex-dependent differences in these mouse inbred strains as they were previously established in the ENU screening program (4).
Since the development, regulation and elicitation of allergen-specific immune responses involves a large number of different genes, measurement of allergen-specific IgE antibodies and T cells after in vivo allergen challenge is a sensitive screen to obtain information regarding the functionality of the immune system in mutant mice (Fig. 1). For the purpose of in-depth screening of MMLs with abnormal IgE levels, we have developed a model of allergic sensitization and inhalative allergen re-exposure (challenge): Mice are sensitized via the i.p. route with the model allergen ovalbumin (OVA) together with the Th2-adjuvant aluminium hydroxide (alum). As read-outs of the magnitude of the general and organ-specific allergic response, the following established tests and parameters are used in a routine way: serology [OVA-specific (s) IgE, sIgG1, sIgG2a], bronchial airway hyperreactivity (methacholine challenge after allergen re-exposure), allergen-specific T cell reactivity (CD4+ and CD8+ T cells) and cytokine responses (PCR and ELISA), analysis of airway eosinophilia (BAL, bronchoalveolar lavage), skin test for IgE reactivity (active or passive cutaneous anaphylaxis). With the help of these techniques, a complete allergy fingerprint of mutant mice exhibiting an allergic phenotype can be provided during secondary and tertiary screening procedures.
Our laboratory at the Department of Dermatology and Allergy TUM is recognized for its expertise in the analysis of IgE detection methods and of immunologic mechanisms involved in allergic, infectious and autoimmune diseases. Among the many collaborations with other institutes in Europe and the USA (5-10), the close collaborations with the University of Hamburg (Dept. of Biochemistry and Molecular Biology, Prof. R. Bredehorst) is of special relevance for further technical development of allergy screening methods. Mechanisms and the genetics of atopy and allergy is a major research topic of both the Department of Dermatology and Allergy (Head: Prof. J. Ring) and the closely attached Center of Allergy and Environment ZAUM (Head: Prof. H. Behrendt), which is the umbrella organization for the different research groups active in the GMC and ENU murine IgE screening programs. The ENU IgE allergy screen was established by PD T. Jakob (ZAUM). The Department of Dermatology and Allergy TUM is one of the leading allergy centers in Germany with 76 in-patient facilities and a large outpatient clinic and has been established as one of eight national “Atopic Eczema Academies” in the National Consensus Program of the German Health Ministry in the development of schooling programs for eczema in children and adults. Also located at the Department of Dermatology and Allergy TUM is the Clinical Research Division of Molecular and Clinical Allergotoxicology (Head: Prof. M. Ollert), one of the five German national centers of excellence in allergy research funded by the German Ministry of Education and Science (BMBF). The ZAUM brings together different disciplines to study the influence of environmental factors upon development, elicitation and maintenance of allergic reactions. In the 6th framework of the European Union, both institutions have been established as “Centre of Excellence for Allergy” in the European Network for Centres of Excellence “Global Asthma and Allergy European Network GA2LEN”.
Novel allergic phenotypes with relevance to human pathology
The GMC Allergy Screen has so far analyzed more than 40 already established MMLs, which were originally produced with the intention to represent models for various human pathological conditions other than allergy. Most interestingly, for seven of the MML we were able to assign new allergic phenotypes. These included changes in the level of total plasma IgE and in the induction of allergic immune responses.
We also were able to perform in-depth analysis of several newly-identified ENU mutants with altered IgE levels. Some of them are promising candidates for further exploring the underlying mechanisms of allergic diseases.
In an effort to optimize secondary and tertiary screening procedures for allergy mutants, we determined the importance of the dose of allergen administration and the number of aerosolized allergen exposures in modulating the physiologic, inflammatory and immunologic features characteristic to allergen-induced airway inflammation. This approach also helped to optimize time requirements for the sensitization protocol. A short-term protocol eliciting a solid allergen-specific IgE response together with airway eosinophilia and hyperreactivity was established that appears to be more suitable to analyze large numbers of animals in the search for allergic phenotypes which are not obvious under baseline conditions (Javaheri et al., in prep.). The use of allergen challenge conditions in the search for allergic phenotypes is of clinical relevance, since the importance of allergen-specific IgE in the absence of elevated total IgE has also been reported in humans with atopic allergic disorders (9).
Outlook
The assay systems developed so far for the primary and secondary Allergy Screen at the GMC have proven to be sensitive and reproducible, and as already demonstrated by the first results, the methods were able to identify new allergy phenotypes in MML. In the primary allergy screen, MMLs are routinely examined on the basis of their total plasma IgE levels. Future directives for the primary allergy screening also include the analysis of Fc receptor expression on peripheral blood leukocytes.
Primary screening is performed in close coordination with the other screens at the GMC. According to the established workflow of the GMC, up to 26 MML will be analyzed per year. The results of these analyses will be discussed with the mouse provider and a decision on a potential further in depth analysis is made based on the primary data. For each MML, a comprehensive summary of all data is provided.
In conclusion, the establishment of a successful phenotyping platform for MMLs to detect new phenotypes with relevance for IgE-mediated allergic diseases marks an important step forward. The phenotyping approach has already proven its ability to define new disease models for allergy that can be provided to the scientific community. This will ultimately lead to the discovery of new genes involved in the humoral and cellular regulation of IgE and IgE-mediated diseases, and subsequently to new therapeutic strategies in the treatment of allergy.
Lit.: 1. Leung DYM and Bieber T. Atopic dermatitis. Lancet. 2003;361:151-160. 2. Ring J et al. Why are allergies increasing? Curr Opin Immunol 2001;13:701-708. 3. Cookson WO and Moffat MF. The genetics of atopic dermatitis. Curr Opin Allergy Clin Immunol 2002;2:383-387. 4. Alessandrini F. et al. ENU mouse mutagenesis: Generation of mouse mutants with aberrant plasma IgE levels. Int Arch Allergy Immunol. 2001; 124:25-28. 5. Köllisch G et al. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology 2005; 114: 531-541. 6. Weidinger S et al. Association study of mast cell chymase polymorphisms with atopy. Allery 2005; in press. 7. Gutermuth J. et al. Mouse models of atopic eczema criticaly evaluated. Int Arch Allergy Immunol 2004; 135: 262-276. 8. Pfister H. et al. Anti-neutrophil cytoplasmic autoantibodies (ANCA) against the murine homolog of proteinase 3 (Wegener's autoantigen) are pathogenic in vivo. Blood 2004; 104: 1411-1418. 9. Ollert MW et al. Allergen-Specific IgE Measured by a Continuous Random-Access Immunoanalyzer: Interassay Comparison and Agreement with Skin Testing. Clin Chem 2005; 51: 1241-1249. 10. Grunwald T. et al. Molecular cloning and expression in insect cells of honey bee venom major allergen acid phosphatase (Api m 3). J Allergy Clin Immunol 2005 (in press).


