Introduction
Cardiovascular diseases (CVD) remain the most prevalent disorders and leading cause of death. CVD burden results from coronary artery disease leading to myocardial ischemia and infarction, contractile dysfunction due to cardiomyopathy, myocardial damage or overload, and electrical disorders that may occur as primary channel defects or as a consequence of myocardial damage. In aggregate, these mechanisms lead to progressive congestive heart failure and sudden cardiac death. In the individual patient and also in affected families these disease phenotypes are coexistent and closely interdependent. In comparison, the analysis of animal models allows to focus on individual phenotypes of CVD and to study their development from the outset. Moreover, targeted genetic manipulation of the experimental organism enables direct testing of putative disease genes under standardized environmental conditions.
The mouse model is recognized as an essential tool for CVD research, because its physiology compares well to the human counterpart. Invasive manipulations and (electro)physiological measurements are now possible in the mouse due to recent advances in microsurgery and technology. Detailed cardiovascular and electrophysiological phenotyping used to be done in rat models. Our group reported a rat model expressing human truncated cardiac troponin T molecules identified in patients with familial hypertrophic cardiomyopathy. The phenotype of this rat model mirrored the human disorder excellently including the sudden death due to severe ventricular arrhythmia (1). We will examine next a novel mouse model with a targeted mutation (knock-in) in deltal-sarcogylcan, which we identified in a family with dilative cardiomyopathy. Thus, the study of human mutations in the mouse does not only prove their clinical relevance, but may also reveal their mechanisms and new therapeutic options.
Results/Project Status
Goal. A novel, primary cardiovascular screen has been introduced recently to endorse the extensive and sophisticated phenotyping program that is already offered at the German Mouse Clinic (GMC). Non-invasive phenotyping includes echocardiography, electrocardiography (ECG), and tail-cuff blood pressure measurements embedded in the regular work flow of the GMC. Thus, cardiovascular phenotyping will not be limited to mere measurements of cardiac function. Collaborating pulmonary and metabolic screens contribute important information to complement the picture of the cardiovascular functional capacity of a mouse model.
Specific Aims. The first specific aim of the cardiovascular screen is focused on translational research by identification of novel candidate genes relevant for human CVD: (i) Mutant mouse models with an abnormal cardiovascular phenotype will be further characterized in secondary and tertiary screens to unravel the underlying disease mechanisms. (ii) Additional protein-protein interaction studies will identify novel proteins supporting the dissection the complete disease pathway. (iii) These candidate genes will be also screened for mutations in large patient cohorts and families to confirm their relevance for human disease. (iv) Finally, specific therapies can be tested immediately in these mouse models.
The second specific aim is focused on cooperative research by promotion of networks and collaborations to improve the usage of all the GMC phenotyping facilities: (i) Informations on the mutant mouse models and their cardiovascular phenotypes will be summarized and updated regularly on the web site of the GMC (http://www.mouseclinic.de) and the CVD network (http://www.herz-kreislauf-netz.de). Progress reports are given at national (e.g. the Annual Meeting of the German Society of Cardiology) and international meetings (eg. the Mouse Genome Conferences, Conferences of the Complex Trait Consortium). (ii) The NGFN disease network for CVD will use the facilities at the GMC to obtain a broad phenotypic characterisation (not only cardiovascular!) of targeted gene mutants that are produced in the CVD network.
Methods. The primary cardiovascular screen has been implemented just recently including state-of-the-art appliances and protocols (SOPs) similar to those employed in other large phenotyping programs, eg EUMORPHIA (http://www.eumorphia.org/EMPReSS/servlet/EMPReSS.Frameset) and the Phenome Project, a PGA of the NIH-NHLBI at the Jackson Laboratories (www.jax.org/phenome/). Training of staff and continuous monitoring of quality is carried out in collaboration with members of the Mouse Clinic at the University of Muenster, Germany (P. Kirchhof, J. Stypmann).
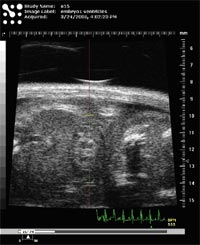
Heart anatomy and function are visualized in real time by echocardiography under sedation (Isoflurane) using a Vevo 660 system (VisualSonics Inc., Toronto) with pulsed wave doppler capacity. Equipped with a 45-MHz ultrasound probe even the development of the fetal heart can be visualised (Fig. 1).
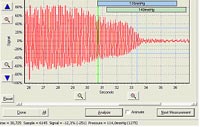
Heart rhythm is assessed under sedation using a digital ECG (EMKA Technologies, Paris) allowing highly sophisticated and semi-automated ECG trace analysis (Fig. 2).
.jpg)
Blood pressure is measured using tail-cuffs on conscious mice and the MC4000 multichannel platform (Hatteras Instruments Inc., Cary N.C.). Repeated measurements are obtained after a short training period to accustom the mice to the procedure (Fig. 3)
Results. After establishing technical appliances and methods we provided proof of principle examining several common inbred laboratory mouse strains (C57BL/6J, C3HeB/FeJ, 129S1/SvImJ, BALB/cByJ) and a wild-derived inbred strain (PWD/Ph). Apart from strain-dependent differences we identified both sex- and age-dependent differences in blood pressure and ECG parameters. Preliminary analysis confirmed previously published findings (www.jax.org/phenome/). Reaching the next milestone as planned we have now joined the regular work flow of the GMC to examine mutant mouse models.
Outlook
The cardiovascular screen is a dedicated NGFN networking project of the CVD network and the SMP models. It will not only promote collaborations and knowledge transfer among the participating scientists, but also provide new opportunities for research networking on a larger, international scale. Presentations of the CV screen at international workshops have been met great interest. SOPs are exchanged and discussed with members of the Phenome PGA in order to conform them.
Bringing together clinical scientists and mouse geneticists in the interdisciplinary setting of the GMC will improve considerably the characterization of precious mouse models and add to the overall depth of phenotyping in the GMC.
Lit.: 1. Frey N et al. Transgenic rat hearts expressing a human cardiac troponin T deletion reveal diastolic dysfunction and ventricular arrhythmias. Cardiovasc Res. 2000 Aug;47(2):210-1. 2. Gailus-Durner V. et al. Introducing the German Mouse Clinic: open access platform for standardized phenotyping. Nat Methods. 2005 Jun;2(6):403-4.


