Introduction
According to the World Health Organization (WHO), lung diseases are among the leading causes of death worldwide. Morbidity and mortality from lung diseases has steadily increased in recent years. Major contributors are chronic obstructive pulmonary diseases (COPD) and asthma, both of which are known to be determined by a complex interplay of inherited predispositional and environmental factors. Despite considerable scientific efforts and the development of various animal models, a substantial gap still remains between those gene-phenotype interactions presumed to play a role in patients suffering from asthma and COPD, and available mouse models for testing the significance and interplay of genetic variants and for developing new therapeutic strategies in biomedical research.
Disorders of respiratory control are also of medical relevance, especially those occurring in infants (2,3,4,5,6,). Despite our high social and economic standards, Germany is one of the “leading” countries in Europe for the incidence of fatal sudden infant death syndrome (SIDS). SIDS is allegedly a result of abnormalities in the lower brain stem. To improve our current understanding on genetically linked pathways for disorders of central respiratory control, advanced animal models have to be developed from the phenotyping of mutant mice.
The Respiratory Lung Function screen aims at the detection of so far unknown respiratory phenotypes in mutant mice, with a focus on their association to various lung diseases and disorders of central respiratory control (7,8,9,10). Identification of functionally relevant gene-phenotype associations will substantially improve our pathophysiological understanding and provide new animal models for experimental research and the development of specific therapeutic strategies.
Project Status
The lung function screen as a part of the `primary screen´ workflow of the GMC is applied to assess alterations of breathing pattern in mutant mouse lines. More detailed analyses of lung function are provided by `secondary´ and `tertiary´ screen for research in cooperations with other labs.
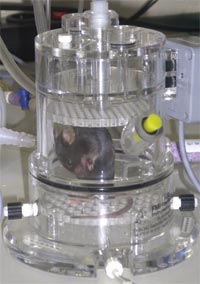
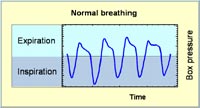
Primary screen Breathing patterns in unrestrained animals are assessed by whole body plethysmography (1,6, Fig. 1). Pressure changes (Fig. 2) due to inspiratory and expiratory temperature and humidity fluctuations during breathing are measured over 40 minutes and transformed into flow and volume signals, so that automatic data analysis provides tidal volume, respiratory rate, minute ventilation, inspiratory and expiratory time, as well as peak inspiratory and peak expiratory flow rates. Specific tidal volumes and minute ventilations are calculated from the respective absolute values in relation to the body weight of the animals. Consequently, the technique provides a whole bunch of parameters for the assessment of specific alterations in breathing. Moreover, our SOPs allow to measure these parameters at typical levels of activity, i.e., during phases of sleep, of rest, or of activity.
Secondary screen Our unit provides lung function testing comparable to that available in pulmonary divisions. Measurements are carried out in anesthetized, intubated and mechanically ventilated animals (8,9,10). The unit allows the measurement of lung size (total lung capacity) and all volumetric subunits of the lungs including the volume of the conducting airways. Respiratory resistance, static and dynamic lung compliances as well as intrapulmonary gas mixing and alveolar-capillary gas transfer are other parameters delivered by the unit. Our unit has been applied to study sex and inter-strain differences of lung functions in various inbred mouse strains and selected mutant lines.
Tertiary screen SOPs for bronchial challenges in mice were established to characterize the degree of airway reactivity. For the measurement in unrestrained animals, whole body plethysmography is applied and changes in the parameter “enhanced pause” (penh) is used to assess the level of airway reactivity. The protocol consists of a determination of baseline values and a negative control with buffered PBS. Then, five, progressively increasing concentrations of a methacholine aerosol are delivered to the animals to set up a precise dose-response curve. For a direct measurement of changes in airway resistance in response to a challenge, the above introduced lung function unit is used. The respective protocol covers the same steps as mentioned above, but methacholine is intravenously infused to guarantee that the different doses of methacholine are administered with highest precision.
The mouse strains and mutant lines are provided by other labs of the NGFN consortium or originate from the ENU mutagenesis program at the GSF. The lung function laboratory contributes basic measurements for disorders of lung function and respiratory control in the mouse model.
Results
The screen aims at the detection of so far unknown respiratory phenotypes in mutant mice and the identification of genes involved in various lung diseases and disorders of central respiratory control. In the last years we studied 37 mutant lines through the coordination of the GMC. In 18 of these mutant mouse lines (MML) new, so far unknown phenotypes were detected with suggestive or clear lung disorders. Some of our findings are summarized below:
MML1 ENU-generated MML displaying a Parkinson-like bahavior: Mutant mice exhibited a significantly different breathing pattern at all levels of activity. This finding was principally reflected in a different behaviour of body weight-specific minute ventilation: Starting from comparable values at sleep, mutant mice showed a stronger increase in ventilation with increasing levels of activity than did control mice. This suggests an enhanced oxygen demand in active mutant mice than in their control counterparts. Another major and possibly correlated finding of the screen is the observation that mutant mice are less active than control animals.
MML2 ENU-generated MML showing an abnormal teeth phenotype: Mutant mice showed significantly lower absolute tidal volumes and minute ventilation accompanied by lower flow rates compared to control mice during rest. One explanation is that mutants have proportional smaller lungs than control mice. During activity compensation via higher breathing rates was more effective in males than in females. This suggests that breathing pattern in females is more affected by the mutation than in males. Since compensation to a certain degree is possible, alteration in breathing pattern in mutants could be a secondary effect due to neurological or muscular alterations.
MML3 ENU-generated MML showing an abnormal teeth phenotype different from MML3: No differences in breathing were found among female animals. Among male mice, however, mutants showed significantly higher breathing rates and lower values for tidal volume compared to control mice. Thus, the mutation seems to affect spontaneous breathing pattern specifically in male mice. This is supported by the observation that unusual sex differences were detected in mutant mice.
MML4 ENU-generated MML with progressive deafness: The main difference between control and mutant mice is the different level of activity. Mutants were mostly active during the measurement period and were breathing at rates between 400 and 600 breaths per minute, while control mice showed an extended phase of rest and even a sleeping phase. The observed differences in breathing pattern are most likely related to the different activity behaviour.
MML5 knock out of a gene highly expressed in dopaminergic neurons: These mice breathed at comparable respiratory rates, but with smaller tidal volumes than their control counterparts at both levels of activity. As a result, minute ventilation was about 20% lower in mutant mice. We hypothesize that this finding may be related to altered lung functions, differences in CNS control of ventilation and/or peripheral chemoreceptor sensitivity. Further lung functions tests are required to illuminate the pathophysiological basis of the observed differences (Publication in preparation).
MML6 knockout of a gene coding for a protein implicated in the formation and maintenance of myelin: In general, mutant mice showed significantly lower breathing rates and consequently a significantly higher inspiratory and expiratory timing compared to control mice During rest, lower breathing rate is compensated by higher tidal volumes in the mutants resulting in comparable minute ventilations. This compensation is much less sufficient during activity. The results suggest disturbances in the respiratory control of breathing.
MML7 knock out of a gene coding for a multifunctional enzyme of the fatty acid metabolism: Gender specific differences were detected in this line. At both rest and activity, MFP2 females showed considerably higher specific tidal volumes and minute ventilations than did female wild types. In connection with the well known growth retardation, this observation suggests an impaired respiratory lung function and/or a higher oxygen demand. For MFP2 males, the most striking finding was their small tidal volume during both rest and activity. Taken together with the observed longer expiratory times and lower expiratory flow rates, one may hypothesize that MFP2 males have smaller and perhaps less compliant and more resistive lungs than their wild-type counterparts. This impairment could again be primarily lung related due to growth retardation, or it could be a secondary effect due to co-existing malformations of the vertebral column, which were detected in the Dysmorphology Screen
Inbred strain specific phenotype variance of lung function parameters among common laboratory mouse strains was determined and a great diversity detected among strains. The worldwide first whole genome scan for pulmonary function in mice revealed complex heritable traits for lung function. Significant linkages with LOD (logarithm of the odds) scores up to 6.0 were detected on chromosomes 5, 15 and 17, and 19.
Outlook
Currently, secondary screens are conducted in some of these novel mutant lines to rule out possible pathophysiological pathways and supply the mouse provider a comprehensive view of respiratory findings. Based on these findings, mutant lines may be considered as novel models for lung diseases. The primary screening through the coordination of the GMC will be carried on.
Lit: 1. Drorbaugh J.E. and W.O. Fenn (1955): A barometric method for measuring ventilation in newborn infants.Pediatrics 16: 81-87. 2. Katz D.M. (2003): Neuronal growth factors and development of respiratory control. Respir. Physiol. Neurobiol. 135: 155-165. 3. Mead J. (1960): Control of respiratory frequency. J. Appl. Physiol. 15: 325-336. 4. Otis A.B., W.O. Fenn and H. Rahn (1950): Mechanics of breathing in man. J. Appl. Physiol. 2: 592-607. 5. Tankersley C.G. (1999): Genetic control of ventilation: What are we learning from murine models? Current Opinion in Pulmonary Medicine 5: 344-348. 6. Tankersley, C.G., R.S. Fitzgerald, R.C. Levitt, W.A. Mitzner, S.L. Ewart and S.R. Kleeberger (1997): Genetic control of differential baseline breathing pattern. J. Appl. Physiol. 82: 874-881. 7. Reinhard C., G. Eder, H. Fuchs, .Ziesenis, J. Heyder and H. Schulz (2002): Inbred strain variation in lung function. Mammalian Genome 13: 429-437. 8. Schulz H., C. Johner, G. Eder, A. Ziesenis, P. Reitmeier, J. Heyder, R. Balling (2002): Respiratory mechanics in mice: Strain and sex specific differences. Acta Physiol. Scand 174: 367-375. 9. Yamaguchi S., A. Balbir, B. Schofield, J. Coram, C.G. Tankersley, R.S. Fitzgerald, O´Donnell C.P., Shirahata M. (2003): Structural and functional differences of the carotid body between DBA/2J and A/J strains of mice. J. Appl. Physiol. 94: 1536-1542. 10. C. Reinhard, B. Meyer, H. Fuchs, T. Stöger, G. Eder, F. Rüschendorf, J. Heyder, P. Nürnberg, M. Hrabé de Angelis, H. Schulz (2005): Linkage analysis identified multiple genetic loci for lung function in mice. Am. Rev. Respir. Crit. Care Med. 171: 880-888.


