Introduction
Pain is an important signal alerting us to the possibility or occurrence of tissue injury. Chronic pain, however, is a pathological condition that does not serve any useful purpose, but results in a rather substantial loss of life quality. Indeed, chronic pain is an, often debilitating, disorder and constitutes one of the most common and costly health problems, particularly in the elderly. Since virtually all known pharmacological targets for pain management have been saturated with small molecule agonists or antagonists, novel pain therapies depend on the identification of novel drug targets.
In recent years, mouse mutants, most of which have been generated by gene targeting, have become an increasingly powerful tool for the analysis of nociceptive signalling. Mouse models have been established that show alterations at virtually all levels of pain perception (Fig. 1). Mutants include mice with developmental defects in nociceptive neurons, in which primary nociceptive afferents do not respond appropriately to painful stimuli (1), with defects in the central processing of pain, with disruptions of the descending modulation of pain signalling (1), as well as mutants in which dynamic adaptation of the nociceptive systems under pathological conditions are altered (2). These mouse strains have enabled researchers to study these different aspects of pain signalling at the molecular level and to evaluate the mechanisms of action of analgesic drugs.
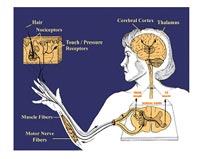
We screen mouse mutants in the German Mouse Clinic for phenotypes related to pain signalling. The goal of this screen is to first identify mice with altered pain responses in a sensitive primary screen. Those strains that were identified as being hyperalgesic or hypoalgesic will then be subjected to in-depth pharmaco-behavioral studies in a wide range of animal models of nociception.
Project Status
The nociceptive screen is performed in three steps. A ‘primary screen’ is part of the standard phenotyping program at the GMC. The more detailed ‘secondary’ and ‘tertiary screen’ are based on the results of the primary screen and are prepared for research co-operations with other labs.
Primary Screen
We use a slightly modified version of technique of Eddy and Leimbach (3) as previously described (4, 5). Briefly, the mice are placed in the hot plate apparatus (TSE GmbH, Germany) on a metal surface maintained at 52 ± 0.2#C. Locomotion of the mouse on the hot plate is constrained by 20 cm high Plexiglas wall to a circular area with a diameter of 28 cm. Mice remain on the plate until they perform one of three behaviours regarded as indicative of nociception: hind paw lick, hind paw shake/flutter, or jumping. We use only hind paw responses, because fore paw licking and lifting are components of normal grooming behaviour. Each mouse is tested only once since repeated testing can lead to latency changes (6). This test is performed in the GMC.

Secondary screen
The aim of the secondary screens is to determine whether altered pain responses of mutant animals were caused by defects in primary nociceptive neurons, or at the level of the spinal cord, or whether it involved supraspinal mechanisms. For this purpose, we employ a variety of specific nociceptive paradigms. The tail flick test, for example is an assay to evaluate pain reflexes that are controlled at the level of the spinal cord. It was originally developed by D`Amour and Smith (7). When the animals’ tail is exposed to a radiant heat stimulus, a withdrawal reflex (a vigorous flexion of the tail) is elicited when the stimulus reaches the nociceptive threshold. A similar withdrawal reflex can be triggered at the paw with a focussed light beam (Hargreaves test; 8). At this level we also assess the animals’ responses to inflammatory pain using chemical irritants (9 - 11).
Finally, we are determining inflammation-induced hyperalgesia and allodynia produced by carrageenan. (12, 13). Mice were tested for thermal sensitivity of both hind paws on the test of Hargreaves (14).
Tertiary Screen
At the third level chronic pain responses were evaluated. For this purpose, the Chung peripheral nerve injury model is used (15). In the tertiary screen, we also evaluate animals’ responses to analgesic drugs.
Results
Since the beginning of the German Mouse Clinic (GMC) the core facility provided 37 mutant mouse lines and six inbreeding or hybrid lines for the primary nociceptive screen. Among those, we already found 10 strains with significantly altered pain reactivity. This is a surprisingly high number and might reflect a bias in the mouse mutants for genes involved in signal transduction. We do not believe that nociceptive phenotypes are particularly common among mouse mutants, because we did not find an over-representation of pain mutants in an ENU mutagenesis screen (16).
Included in these strains is a mutant mouse lines that has been established in our lab (Asic3-knockout; 17). We had already tested this mouse strain comprehensively for pain phenotypes. These previous results suggested to us that we might find a very mild hyperalgesic phenotype under the experimental condition of the primary screen. Indeed, we were able to verify such a phenotype, thus demonstrating the validity of our testing conditions.
Some of the phenotypes discovered in this screen are extremely interesting. For example, we found one mouse strain in which female mutant animals were hypoalgesic, while male mutants were hyperalgesic. To the best of our knowledge, such a sex-specific inversion of a knockout phenotype has never been described before. Other mutants have implicated novel regulatory pathways in nociceptive signalling. It will now be important, and shall be one focus of the next phase of this project, to validate and further extend the analysis of these mouse strains in secondary and tertiary nocicpetive tests, which include behavioural animal models for inflammatory hyperalgesia and allodynia, as well as animal models for neuropathic pain.
Outlook
Novel and interesting mouse mutants with altered nociceptive responses have been identified in a primary screen in the GMC. The first of these mutants have now entered the secondary and tertiary screening phase, thus providing a comprehensive phenotypic characterization of pain-related responses. We expect that some of these mutants will be interesting novel animal models, for clinical pain conditions. With the help of these animals, we will learn more about the regulation of pain sensation and the pathopysiology of chronic pain disorders. The ultimate goal of this research is the development of novel pain treatments and novel analgesic drugs. There are already a number of novel drugs in clinical trials, which have been developed based on findings in genetic animal models. Thus, this line of research may help to improve to quality of live for the many million of people who suffer from debilitating pain disorders.
Lit.: 1. Agarwal, N. et al. Conditional gene deletion in primery nociceptive neurons of trigeminal ganglia and dorsal root ganglia Genesis 38 (2004) 122-129. 2. Harvey, R.J. et al. Gly #3: An essential target for spinal PGE2-mediated inflammatory pain sensation Science 304 (2004) 884-887. 3. Eddy, N.B. and Leimbach, D., Synthetic analgesics II. Diethienylbutenyl- and dithienylbutylamines, J. Pharmacol. Exp. Ther., 107 (1953) 385-393. 4. Zimmer, A. et al. Increased mortality hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knock out mice, Proc. Natl. Acad. Sci. USA 96 (1999) 5780-5785. 5. Zimmer, A. et al. Hypoalgesia in mice with a targeted deletion of the tachykinin 1 gene, Proc. Natl. Acad. Sci. USA 95 (1998) 2630-2635. 6. Mogil, J.F. et al. Heritability of nociception I: Responses of 11 inbred mouse strains on 12 measures of nociception, Pain 80 (1999) 67-82. 7. D`Àmour and F.E. Smith, D.L. A method for determining loss of pain sensation J. Pharmacol. Exp. Ther. 72 (1941) 74-79. 8. Hargreaves, K. et al.. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia Pain, 32 (1988) 77-88. 9. Hendersoth, L.C. and S. Forsaith. Antagonism of the frequency of phenylquinone-induced writhing in the mouse by weak analgesics and nonanalgesics J. Pharmacol. Exp. Ther. 125 (1959) 237-240. 10. Siegmund, E. et al. A method for evaluating both non-narcotic and narcotic analgesics Proc. Soc. Exp. Biol. Med. 95 (1957) 729-731. 11. Koster, R. et al. Acetic acid for analgesic screening Fed. Proc. 18 (1959) 412. 12. Winter, C.A. et al. Carrageenin-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs Proc. Soc. Exp. Biol. Med. 111 (1962) 544-547. 13. Tonussi, C.R. and Ferreira, S.H. Rat knee-joint carrageenin incapacitation test: an objective screen for central and peripheral analgesics Pain, 48 (1992) 421-427. 14. Dirig, D.M. Isakson, P.C. Yaksh, T.L. Effect of COX-1 and COX-2 inhibition on induction and maintenance of carrageenan-evoked thermal hyperalgesia in rats. J. Pharmacol. Exp. Ther. 285 (1998) 1031-1037. 15. Kim, S.H. and Chung, J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50 (1992) 355-363. 16. Martin Hrabé de Angelis et al. Genome-wide, large-scale production of mutant mice by ENU mutagenesis Nature Genetics 25 (2000) 444-447. 17. Chen, C.C. et al. A. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci U S A. (2002) 99 8992-8997.


