Introduction
Variation in clinical chemical and haematological values of the laboratory mouse occurs due to multiple genetic as well as environmental factors. In humans, most inherited metabolic disorders lead directly or indirectly via altered organ functions to changes of these diagnostic laboratory parameters. Identification of mutant mice in phenotype- and/or gene-driven approaches showing similar clinical chemical deviations provides novel appropriate animal models for the detection of the causative mutation and the pathologic consequences thereof (1). For example several mouse models for human diabetes type2 have been developed (2). As homeostasis of clinical chemical parameters is regulated by polygenic factors, quantitative trait loci (QTL) affecting chosen parameters and influencing disease susceptibility are revealed using mouse strains which show considerable differences in the parameter in question. Further phenotypic and genetic analyses contribute to the integrated evaluation of the mammalian genome function (3) and systems biology of complex organisms.
In the clinical-chemical and haematological examination at the GMC genotype related organ defects, metabolic or haematological disorders are revealed in mutant mouse lines submitted for the primary screen. We also analyze mutant mouse lines established within the Munich ENU mouse mutagenesis project (4) and samples provided by various collaboration partners within and outside the NGFN. In mouse lines harbouring a homogenous genetic background also genetic effects influencing metabolism or organ function without causing pathological changes can be detected. Additionally we are involved in the Europe-wide efforts to standardize mouse phenotyping and phenotypically characterize mouse inbred strains.
Results/Project Status
Equipment and methods used
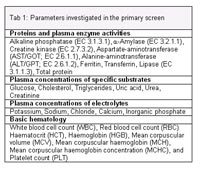
The clinical chemical and haematological screening procedures are performed according to the high-throughput techniques carried out on human samples including the same technical equipment and reagents (5). We use an Olympus AU400 autoanalyzer (Olympus, Hamburg, Germany, Fig. 1) for the determination of 20 different clinical chemical parameters including enzyme activities, as well as concentrations of specific substrates, electrolytes and plasma proteins (primary screen). Additionally eight basic haematological parameters are analyzed using an ABC animal blood counter (Scil, Viernheim, Germany, Fig. 2), which has been validated for the analysis of mouse blood by the manufacturer (Table 1). In the secondary and tertiary screens additional clinical chemical parameters, urinary protein excretion (6), glucose tolerance, insulin level and various growth related hormones, coagulation time, differential blood counts and blood gas values are analyzed. Mutant mouse lines examined at the GMC are routinely investigated with the primary screen at the age of 12 weeks. A 500µl blood sample is collected under ether anaesthesia from the retro orbital vein plexus and dived into two fractions (450µl in a Li-heparin coated tube and 50µl in a EDTA coated tube). The clinical chemical analyses are done using an aliquot of 130µl heparin plasma. The remaining plasma and the cell pellet are used by other screens within the GMC. The EDTA blood is used for the haematological investigations.


The primary screen at the GMC
Since the GMC started working in 2002, 41 mutant mouse lines were provided by the core facility for the primary phenotypic analysis in the GMC. All lines were investigated in the primary clinical-chemical and haematological screen. In 31 of these lines genotype specific significant differences of various clinical-chemical and/or haematological parameters have been detected. In some cases, small changes and sex specific phenotypes were detected, which may indicate additional metabolic pathways to be influenced by the mutation investigated. In nine lines a hitherto unknown clinical-chemical and/or haematological phenotype was found: Changes in the white and red blood cell count were detected in three knockout lines and one line with chromosomal abnormalities; the fat and energy metabolism was affected in two ENU-induced mutants showing an abnormal behaviour and one spontaneous mutant identified due to abnormalities of the eye. Beside that two of the latter mouse lines and two other ENU-induced mutants with abnormal tooth colour displayed signs for liver and/or pancreas dysfunction, and another ENU-induced mutant with bone abnormalities had an elevated plasma urea concentrations indicating kidney dysfunction. The results clearly showed the relevance of the mutation investigated for the affected metabolic pathway or organ function. In most of the cases they were supported by the findings of other screens. Manuscripts including some of our findings are currently in preparation. The clinical-chemical screen proved to be successful in the detection of new phenotypes in mutant mice.
The Munich ENU mouse mutagenesis project
Based on our phenotyping of mice derived from the Munich ENU mouse mutagenesis project (7), 33 new mutant mouse lines have been established within this project. We also enabled the maintenance and cryoconservation of several mouse lines established before we took over the analyses in January 2002, by phenotyping the offspring from the respective matings (8). Beside that we have been involved in the chromosomal mapping of the causative mutation of several of these mouse lines, since we did the phenotyping of the N2 backcross mice, which were then used for the linkage analysis. Currently we are doing an in depth phenotypic analysis of selected mouse lines derived from this project in parallel to the molecular investigations to identify the causative mutations.
Activities based on other scientific collaborations
Beside the investigations on mouse lines sent to the GMC, we have been involved in phenotypic investigations of mice belonging to the Munich ENU mouse mutagenesis screen and of several additional mouse lines and also other species in the frame of collaborations. The collaboration partners partly belonging to the NGFN worked in different institutes of the GSF or universities in Munich (LMU, TUM), or belonged to universities or institutions in other German (Berlin, Hamburg, Freiburg) or foreign towns (Fribourgh, Marseille, Gent). We did investigations on a mouse model of polycytaemia vera using mice imported to the GMC and analyses on plasma, serum, urine and other body fluid samples of several mouse lines and induced disease models as well as samples from other animal species (cattle and swine) sent to our lab aiming on a joint publication of the results achieved. Several publications resulting from these activities are currently in preparation.
Eumorphia
We have also been involved in the development of standardized protocols for clinical-chemical and haematological phenotyping of mice, by developing and/or testing the SOPs for the blood sample collection and investigation and the SOPs for glucose tolerance tests in mice in collaboration with the Eumorphia consortium.
Determination of normal values in inbred mice
Using the standardized protocols we determined normal values of all clinical-chemical parameters in plasma and urine as well as a spectrum of haematological parameters we measure for different mouse inbred strains with and without prior fasting of the animals. Most parameters we measure display significant strain and/or sex differences. Figure 3 for example demonstrates typical strain and gender specific differences of cholesterol and triglyceride levels in mice as detected by our screen.
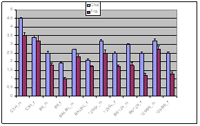
This explains the high variance of our parameters observed in some mouse lines with a mixed genetic background and underlines the necessity of getting the mutations investigated on a defined homogenous genetic background. This can be achieved by backcrossing of the mutated mice to an inbred strain over at least six generations.
Outlook
We try to broaden the parameter spectrum analysed by the clinical chemical screen by testing new parameters for their reliability in mice. Since the blood volume available from a single mice is strictly limited, most of these additional parameters can not be included in the primary screen. They are used to get more detailed informations on the phenotype of selected mouse lines investigated in secondary or teriary screens based on a collaboration with the mouse provider.
Our work will contribute to the elucidation of gene functions and the characterization of new mouse models for human diseases. Especially our current work on ENU induced mutant mouse lines will result in a detailed description of new mouse models for human diseases, displaying defects in haematopoiesis, iron metabolism or kidney function.
Lit.: 1. B. Rathkolb et al.: Exp. Physiol. 2000 85, 635-644. 2. D.L. Coleman 1982 in Foster et al. (eds): The mouse in biomedical research VOL 4, 125-132. 3. Members of the complex trait consortium: Nat. Rev. Genet. 2003, 4, 911-916. 4. B. Rathkolb et al.: Mamm. Genome 2000 11, 543-546. 5. Gailus-Durner et al.: Nat. Methods 2005, 2, 403-404. 6. B. Rathkolb et al.: Nephron Exp. Nephrolog. 2005 100, e143-e149. 7. Hrabé de Angelis et al.: Nat Genet. 2000 25, 444-447. 8. M. Mohr et al.: J. Lipid Res. 2004 45, 2132-2137


