Introduction
The production of mutant mouse lines (MMLs) by gene knockout and transgenic technology or mutagenesis generates a large collection of models for in vivo analysis of distinct gene functions, many of them potentially providing models for the study of human diseases. However, full and efficient use of this large resource is currently hindered by shortcomings in the quality and extent of phenotypic analysis. To overcome the bottleneck of standardized, comprehensive phenotyping in mouse genetics, we established within the National Genome Research Network (NGFN) a unique mouse phenotyping center (German Mouse Clinic, GMC) with open access to the scientific community. In the GMC, experts from various fields of mouse physiology and pathology in close cooperation with clinicians work side by side at one location. Scientists from the Universities of Marburg, Bonn, the Ludwig-Maximilians-University and the Technical University Munich, and the GSF work in the GMC, where the comprehensive primary screen is performed.
The facilities of the GMC were designed to provide an optimized specific pathogen-free (SPF) infrastructure with individual units (laboratories and adjacent mouse rooms) for each screen. The examinations comprise the following areas: allergy, behavior, clinical chemistry, dysmorphology, energy metabolism, steroid metabolism, eye development and vision, host-pathogen interactions, immunology, lung function, molecular phenotyping, neurology, nociception, and pathology. A screen for cardiovascular analyses was recently implemented in collaboration with the cardiovascular network of the NGFN, organized and coordinated by the University of Heidelberg. Additional units are designated to accommodate guest scientists for collaborative work, especially for the transfer of standardized protocols to other laboratories. Detailed secondary and tertiary screens are offered in all areas of the GMC, and supplemented by additional secondary screens for host-pathogen interactions performed at the GBF in Braunschweig.
Results/Project Status
Infrastructure
A specialized building with a modular structure was designed and constructed, combining laboratory rooms and mouse housing areas. The building (specific pathogen-free (SPF) area) comprises eleven units and rooms for shared infrastructure (Fig. 1).
Each unit consists of a laboratory and an adjacent mouse room, which can be run independently with respect to room temperature, air humidity and air pressure. Two units are designated to accommodate guest scientists who can analyze their mouse mutants with the support of the center. The proximity of laboratories and mouse rooms in one building ensures interdisciplinary communication and close interaction between mouse biologists, veterinarians, physicians, pathologists, engineers, computer scientists, technicians and animal care takers. In addition, animals are exposed to the same environmental conditions keeping variability due to heterogeneous housing/sanitary conditions to a minimum
Organization
For the organization of the GMC we have established a management team (Core Facility) consisting of an overall coordinator, a scientific-technical coordinator, a coordinator for the IT-system, a scientist responsible for the result documentation and an assistant. The Core Facility manages the logistics of the whole import of MMLs for the primary screening, starting from the first request of a collaboration partner until a final report of the phenotyping is provided. Weekly meetings of the GMC scientists are organized: In frequent intervals collaboration partners introduce their mouse lines, results from MMLs are discussed and technical information are exchanged. Meetings concerning specific topics like coordination of secondary screens of a certain MML or statistical analysis of data are arranged as well as special days (mouse handling) or training courses.
In addition to the organization and logistics of the primary screening the Core Facility coordinates the workflows for detailed analysis in secondary and tertiary screens. These workflows have to be individually planned for each MML, respectively.
Other tasks of the Core Facility comprise the coordination of the animal management (e.g. mouse husbandry, health monitoring), project-specific adaptation of the IT-system, public relations (e.g. webpage, presentation of the GMC in public), and quality control on different levels (e.g. Standard operation procedures (SOPs), central organization and database). Since beginning of May 2005, a guest scientist from the Austrian genome research program (GEN-AU) stays for half a year in the GMC to learn phenotyping methods which are to be established back in the home laboratory.
Primary screen
Together with all participating scientists, we have developed a standardized workflow for primary screening in the areas shown in figure 2. To elicit the most comprehensive and effective outcome of the measurements we had to compile the requirements of the primary assays in all participating screens and consider statistical and animal rights aspects. Every other week a new mouse line is imported. A cohort of 60 five-week-old animals (all littermates, 15 female mutants and 15 controls, 15 male mutants and 15 controls) passes the following screens in a predetermined order (see Fig 2).
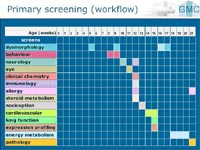
So far, 240 different validated parameters are determined for each MML during one single passage through the GMC. The GMC allows open access to any scientist providing a mutant mouse line for comprehensive phenotyping on a collaborative basis. Therefore, we established a web-based application and mouse import procedure (www.mouseclinic.de). The GMC management team organizes the time schedule for mouse breeding, genotyping, and shipment, as well as details about the implementation of the mutant mouse line analyses into the running phenotyping schedule. To handle the amount of information and deadlines that are connected to each MML we have implemented a specific management database and software. A final report for the mouse provider is compiled of all data and includes a detailed description of all standardized methods and procedures, as well as interpretations from the experts of each screen regarding specific findings. This approach provides a highly efficient workflow for the analysis of up to 26 mutant lines per year.
In general, time slots for mating a new MML to be imported into the GMC are assigned around half year in advance. In few cases, MMLs could not be sent at the planned time point because of breeding problems in the colony. Then, the Core Facility tries to reschedule other MMLs or provides other MMLs or inbred lines for the open slots. Overall, the capacity utilization of the GMC is therefore around 90%. The screening schedule for the primary screen is already quite dense until mid of next year.
Our collaboration partners are members of the NGFN, from European countries like Austria, Belgium, Finland, France, Czech Republic, Italy, Sweden, the USA, and Japan.
Annotation of new phenotypes to mutant mouse lines
Our experiences not only confirmed the feasibility of the general concept of high throughput mouse phenotyping. The obtained results for individual mouse lines also impressively demonstrate that for almost all lines even in well known MMLs new phenotypes were identified (e.g. 715 parameters were altered in 23 MMLs). For example, we have not only confirmed the known pain-related phenotype of a MML mutant, which was provided by the neuronet, but annotated new and unexpected phenotypes in the areas behavior, neurology, clinical chemistry, dysmorphology and energy metabolism. The new insights obtained by the results of the primary screen are the basis for more detailed studies to understand the additional roles of the mutated protein.
We not only detect medically relevant phenotypes in mutants that were established recently. In a MML which was generated 10 years ago and was already analyzed in detail we have annotated an until now undiscovered and not considered immunoglogical phenotype.
To date we have finished the analysis of 40 MMLs in the primary screen (e.g. models for Down syndrome, Mowat-Wilson syndrome or schizophrenia) and six inbred or hybrid lines. Our collaboration partners attend the meetings there we discuss the results of the respective MML. In these meetings we plan the strategies for more detailed analysis of the detected new phenotypes (and select the organs of which RNA expression profiling will be performed). Twenty-six of the screened MMLs are planned for analysis in additional secondary and tertiary studies. In these cases, the collaboration partner has to provide a new group of age- and sex-matched animals. For five MMLs we have finished the secondary screening, for six MMLs the secondary analysis is in progress and for 15 MMLs the secondary screens are in preparation (in many cases the bottleneck is the breeding of the animals). In some cases, the results of the primary screen are already of special interest for the scientific community, and we are preparing manuscripts to publish the data of the primary screen in advance, while the secondary tests are prepared or running in parallel. In the secondary analysis of MMLs all screens of the GMC are involved.
The comprehensive primary screen can also direct the search of new candidate genes (forward genetics). For an ENU mutant, whose causative mutation was not known prior analysis in the GMC, we were able to collect a great deal of information on the basis of diverse phenotypes in the primary screen. This information was used to screen the databases and to dramatically reduce the number of possible candidate genes.

Outlook
The primary screening of MMLs at the GMC was designed for the analysis of mice under resting conditions in a ‘protected’ SPF environment. Consequently, phenotypic alterations especially involved in homeostatic regulation of basic organ and cell functions will be detected during the primary screening. But many biological functions of distinct genes are uncovered only after activation by certain stimuli (e.g. defense mechanism after infection). In addition, multiple environmental factors can trigger the outbreak of specific diseases (e.g. the development of allergic asthma). In order to improve the ability to identify genetically determined phenotypes dependent on environmental factors, our vision for the future is to implement defined challenge test conditions into the workflow for phenotypical analyses of mutant mouse lines at the GMC.
To this end we plan on the operation of a new building for the implementation of defined challenge test conditions (GMC-II).
Lit.: 1. Gailus-Durner, V, Fuchs H et al. (2005). Introducing the German Mouse Clinic: open access platform for standardized phenotyping. Nature Methods 2 (6): 403-404.


