Introduction
RZPD has been founded in 1995 by BMBF to serve as central infrastructure for human genome research in Germany (DHGP). It has been converted to a non-for-profit institute in 2000. Its original task to support German scientists with standardised and quality controlled research material has been expanded considerably. Meanwhile RZPD develops, imports and distributes material for Genomics, Transcriptomics and Proteomics. Moreover it integrates information of all these materials and the experimental results created on their basis. Furthermore, services have been developed on the basis of this materials at RZPD (e.g. robotics, chip analysis, protein expression profiling and certified Affymetrix service).
In the past, serving as the central infrastructure for DHGP and NGFN1, we have envisaged the desperate need of our users to have access to an extensive collection of antibodies directed against human and mouse proteins. Especially the demand for affordable antibody resources is increasing as many research projects focus on multiple proteins at the same time (Haab 2001). The generation of monoclonal antibodies (mainly from mouse) has been done by many commercial entities. However, the process is hampered by high cost and long turn-around times.
In summary, due to lacking sufficient resources of this kind, NGFN1 supported groups could not achieve challenging objectives like dense antibody chips of medium to high throughput diagnostic approaches (e.g. by sandwich assays). Therefore an affordable and available antibody resource needs to be established.
In order to provide antibodies RZPD imports highly characterized monoclonal and polyclonal antibodies from Novagene/Merck. RZPD has mapped these antibodies to genes and integrated this information into the RZPD search and retrieval system GenomeCube™. In these negotiations it became obvious that we will not be able to provide monoclonal antibodies of third parties with substantial price reductions compared to commercial entities.
To overcome these obstacles we intend to establish a fast and efficient technology that has proven its value in the past – the recombinant antibody technology. This technology has shown its capabilities and its commercial value based on therapeutic exploitation of the technology. A major drawback to apply antibody phage display in research was the uncertainty connected with the intellectual property rights on this technology. Therefore RZPD is in contact with commercial patent holders in order to achieve license agreements. Additionally, the expertise of the other projects of the SMP “Antibody Factory” will be included to establish the recombinant antibody technology in a medium throughput manner. We intend to adapt this technology and its application to the ISO9001:2000 standard that RZPD has been granted. The technology will be applied for the isolation of antibodies for third parties, especially for members of NGFN. Great interest has been expressed by many groups. Finally, RZPD will provide selected antibodies for all non-therapeutic applications. The antibody material will be linked to RZPD gene-based web portal to ease the search for specific affinity products. Standard Operation Protocols (SOPs) for their handling will be provided.
Project Status
Contribution to SMP “Antibody Factory”
The RZPD will contribute to the SMP “Antibody Factory” at multiple levels. During the last three years, Research and Development has focussed on the cloning of full open reading frame (ORF) proteins and their expression in E. coli, covering the complete coding sequence of more than 11.000 human genes (Langlais et al. 2004). The DNA sequences of the respective clones have been verified at high quality, and are available in expression ready formats for E. coli, Baculovirus system, yeast and mammalian expression systems. Moreover, we have established more than 130.000 expression tested human cDNA clones representing partial ORFs of the corresponding genes allowing fusion protein expression in E. coli. These expression clones are one important antigen source supplied to the other subprojects of the SMP “Antibody Factory”.
Establishment of antibody phage display at RZPD
In our first work package we aim for robust implementation of the recombinant antibody technology at RZPD Heidelberg in close collaboration with the SMP partners. A strong focus will be set on the standardization of the whole process from antibody selection to the subsequent screening and characterization of the isolated recombinant antibodies. This will allow high reproducibility that is needed if the ultimate objective is to serve third parties.
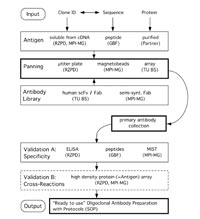
During the first months of this project the antibody phage display technology has been established by panning several antibody phage libraries against several “test antigens”. There is still an ongoing process of optimisation regarding to phage display and screening for antigen specific antibody fragments to enhance the throughput of antibody selections.
For the selection of antigen specific antibody fragments we will use commercial antibody gene libraries as well as antibody gene libraries generated within the “Antibody Factory” consortium. Currently, we negotiate with patent holders in order to achieve freedom to operate with commercial antibody gene libraries. The target antigens for the antibody selections will be defined by the SMP partners. Initial interest has been for a specific set of transcription factors (SMP DNA) and cell signalling cascades.
Automation of antibody selection
RZPD has established and running an extensive lab automation pipeline to manage high throughput applications. Three fully automated liquid handling systems are running in-house, and one will be completely dedicated to the phage antibody selection process. The automation of the antibody selection process for medium throughput will be necessary to achieve the robustness of this process and to meet the demand of antibody selections by collaborating groups. Therefore, we foresee the immediate need to proceed to (semi-)automated adaptation of the whole process. This work package will greatly benefit from expert skills of the RZPD automation engineers and the well serviced robotics (as demanded by ISO9001).
Validation of selected antibodies
The goal of the SMP “Antibody Factory” is the generation of recombinant antibodies by phage display which are applicable in standard immunoassays. Therefore the validation of the obtained antibodies is a very important step and needs to be adapted for the throughput. In the beginning of this project selected recombinant antibodies are tested by ELISA. RZPD proprietary protein arrays covering about 50% of all human proteins will also be used for the validation of the selected antibody fragments. This method allows to identify immediately the cross-reactivity of the recombinant antibodies.
Quality assurances of production processes
Due to its “customers front end” role in the SMP ”Antibody Factory” RZPD is responsible for quality control and assurance. The RZPD puts a major effort into the validation and the verification of material. All full ORF clones offered by RZPD are completely sequenced. The use of these verified materials by many researchers tremendously facilitates comparison of the research results and the subsequent data integration. At RZPD all production processes are in compliance with ISO9001 international quality standards. Each step is documented in and accomplished according to Standard Operating Procedures (SOPs). A barcode system is established for all clone handling. Therefore, each production process can be monitored automatically and is documented.
Infrastructure for the SMP “Antibody Factory”
RZPD will serve as platform for distribution and service within the SMP “Antibody Factory”. RZPD has a well established central database holding all information of genes and partially of proteins in correlation to its materials. We will integrate all information available for antibodies produced in the SMP “Antibody Factory” into the RZPD search and retrieval system (GenomeCube#) to provide this information in a searchable manner to the NGFN2.
Outlook
After establishing recombinant antibody technology at RZPD we will now focus on the automation of the in vitro antibody selection process. Therefore we will optimise the panning protocol as well as the screening of antigen specific antibody fragments in close collaboration with the SMP partners. Now we will adapt these methods to an automated workflow in order to enhance the throughput of antibody selections. For this purpose we will use antigens available within the SMP. Recombinant antibodies against these antigens are being requested by collaboration partners who already showed sincere interest during the initial phase of this SMP. These partners will provide feedback on the selected antibodies, that allows us to optimise the whole procedure and provide the resources in the best manner for third parties. Moreover, we will serve the immediate need for antibodies and will be able to provide extensive application and handling information (inclusive a valuable trouble shooting guide).
Lit.: 1. Langlais, C., Maurer J. and Korn B. Rekombinante Proteine durch “full ORF-Klone”. transkript. 2004;10(1-2):30. 2. Haab, B. B. Advances in protein microarray technology for protein expression and interaction profiling. Curr Opin Drug Discov Devel. 2001 Jan;4(1): 116-23.


