
Introduction
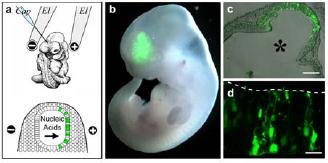
Fig 1: Delivery of nucleic acids into neural stem cells. a) Cartoon showing the injection of nucleic acids into the neural tube (top) and their uptake after electroporation into neural stem cells (green) of the anode-facing side. b-d) Pictures showing the ectopic expression of GFP in neural stem cells (2, 3).
In order to study gene function during embryonic development, systems are required that allow manipulation of gene expression in vivo. However, in mammals, embryonic life is more difficult to analyse because development occurs in utero. An important technological advance in this sense has been the establishment of wholeembryo culture, which allows the development of postimplantation mouse embryos for up to two days in a manner indistinguishable from that of embryos developed entirely in utero. Interestingly, whole-embryo culture can conveniently be combined with various methods of introducing foreign DNA into cells, such as in vivo electroporation (1). For a long time, a major limitation of this technology has been the difficulty to perform loss-of-gene-function studies. We therefore investigated the possibility of combining the technologies of i) in vivo electroporation ii) RNA interference (RNAi) and iii) whole-embryo culture. Our approach uses topical injection followed by directional electroporation of endoribonuclease-prepared small interfering RNA (esiRNA) and whole-embryo culture to knock-down gene expression in a tissue-specific manner in the neural tube, heart, limb and skin (2). Importantly, the extent of targeting can be adjusted by using electrodes of the appropriate size and shape, thus allowing transfection to occur in entire regions as well as in single cells of a given organ (3).
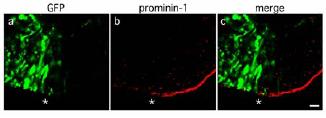
Fig 2: RNAi for prominin-1. Neuroepithelium after coelectroporation of GFP-plasmids, prominin-1-directed esiRNAs and whole-embryo culture. Asterisks indicate the boundary between targeted (left, GFP+) and non-targeted (right, GFP–) cells. Note the reduction of immunoreactivity for prominin-1 in the targeted area (4).
The focus of our research is the understanding of the molecular and cellular mechanisms controlling the onset and the progression of mammalian neurogenesis. In this context, the use of in vivo electroporation and whole-embryo culture offers a unique possibility because embryonic neural stem cells of specific areas of the developing nervous system can easily be targeted by topical injection of nucleic acids into the neural tube followed by directional electroporation (Fig. 1).
The first aim of our project is to better characterise and standardise our approach using the developing neural tube as a model system. RNAi, originally optimised for reporter genes (2), has now been achieved for many candidate genes involved in neurogenesis such as the cell cycle inhibitor Tis21, the stem cell marker prominin-1 (4) (Fig. 2) and others. Silencing of gene expression in the targeted area (identified by the expression of a fluorescent reporter gene co-injected and co-electroporated together with the esiRNAs) was confirmed by in situ hybridisation or immunohistochemistry on cryosections of mouse embryos fixed after 24-48 hours of whole-embryo culture. It is worth noting that RNAi-based approaches become more effective when applied to highly proliferating cells such as neural stem cells. In fact the degradation of the mRNA of a given gene is followed by a dilution of the encoded protein in every consecutive cell division.
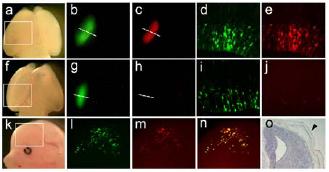
Fig 4: Detection of miRNAs in mouse embryos. a-e) Wholemount (a-c) and sections (d-e) of an E14 brain targeted with a sensor with random sequence (control). f-j) Similar to a-e but using sensor for miRNA-9. k-n) Pictures of an embryo targeted with sensor for miRNA-9 in the skin. o) In situ hybridisation for miRNA-9 in a section through the neural tube and skin.
A second aim of our project is to overcome the temporal limitations of whole-embryo culture. In fact, this system can only be applied for up to 48 hours to embryos between day 7 to 10 of gestation. We therefore established a platform that allows the injection and electroporation of nucleic acids in embryos developing in utero (5). For this purpose pregnant mice are anaesthetised and their uteri exposed. Embryos are then injected into the lumen of the neural tube, electroporated through the uterine walls, and allowed to continue development until completion of gestation (Fig. 3). The efficiency of transfection, in our hands, reaches 20-50% in the targeted area, and about half of the embryos injected and electroporated show expression of the reporter gene in the area of interest. Our next step will be to perform RNAi in embryos developing in utero. Several aspects of this technique will be considered, such as the efficiency of esiRNA as compared to shRNAs in long-term studies of mouse development.
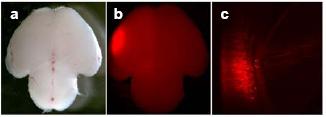
Fig 3: Electroporation in utero. a-b) Pictures of a postnatal mouse brain obtained after injection of RFP-encoding plasmids and electroporation performed in utero at E12. c) coronal section through the targeted area shown in b.
Both artificially delivered siRNAs and physiologically expressed microRNAs silence gene expression by using the same molecular machinery. To better understand the function of miRNAs in development, systems are required that allow their detection in tissues and single cells of living organisms. Recently, sensors for miRNAs have been developed that allow their detection in living organisms. This approach is based on the use of a reporter gene fused to a sequence which is complementary to the candidate miRNA. The expression of the candidate miRNA will therefore trigger the RNAimediated degradation of the mRNA for the reporter, whereas in its absence the reporter is expressed (6). However, in mammals, the major limitation of this technique is that it relies on the generation of transgenic mice. We therefore investigated the possibility of delivering plasmid DNA encoding for sensors into neural stem cells of mouse embryos by in utero electroporation. Importantly, in order to identify cells in which the sensor has been delivered, we used a dual, fluorescent reporter (GFP) plus fluorescent sensor (RFP), plasmid in which both genes are under the control of two identical promoters. This approach allowed us to detect the expression of miRNAs by the absence of RFP in targeted, GFP-positive cells (Fig. 4) (De Pietri Tonelli et al. manuscript in preparation).
Outlook
The use of topical injection and directional electroporation of nucleic acids (reporter genes - functional genes – siRNAs – sensor DNAs) in mouse embryos developing in culture or in utero is a very efficient, fast and cheap approach that allows several kinds of studies such as lineage tracing experiments, manipulation of gene expression and detection of microRNA in a region- and tissue-specific manner. We find it likely that the combination of these approaches will allow a better understanding of the molecular mechanisms controlling fundamental processes of mammalian development.
Lit.: 1. Osumi N and Inoue T. Gene transfer into cultured mammalian embryos by electroporation. Methods. 2001 May 24:35-42. 2. Calegari F et al. Tissue-specific RNA interference in postimplantation mouse embryos with endoribonuclease-prepared short interfering RNA. Proc Natl Acad Sci USA. 2002 99:14236-40. 3. Buchholz F et al. RNA interference in postimplantation mouse embryos. In: Appasani, K. (ed.) RNA interference technology: from basic biology to drug development. Cambridge University Press. 2005 pp.207-19. 4. Calegari F et al. Tissue-specific RNA interference in postimplantation mouse embryos using directional electroporation and whole embryo culture. Special issue on RNA interference. Differentiation. 2004 72(2-3):92- 102. 5. Tabata H and Nakajima K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 2001 103(4):865-72. 6. Wienholds E et al. The microRNA-producing enzyme Dicer1 is essential for zebrafish development. Nat Genet. 2003 35:217-8.


