
Introduction
The Wnt/ -cat, FGF and TGF 1/BMP cascades play multiple roles in development and disease. Various dysmorphology phenotypes and a large number of diseases are associated with the disruption or constitutive activation of these signal pathways. Among them are neurodegeneration (1-2), schizophrenia (3), Alzheimer’s disease (4), and diseases associated with migration defects (5), to name only a few. All three pathways are involved in tumour development and metastasis formation of epithelial tumours (the most common type of tumours). The latter requires deepithelialisation of few tumour cells and acquisition of mesenchymal characteristics (epithelial-mesenchymal transition, EMT) (6). Such cells then move with the blood stream or the lymph to new sites and form metastases. The mesenchymal state is transient and therefore this event can hardly be investigated in vivo in patients. Due to this complication EMT of tumour cells is investigated on model cell lines in vitro. EMT, however, is a common process in mouse embryos undergoing organogenesis. Therefore, the mouse embryo offers the possibility to investigate EMT in vivo. Important results obtained in the mouse can then be checked on human tumour cells to elucidate common mechanisms. For instance, one of the key events of EMT is the down-regulation of E-cadherin, which is common for embryos and tumour cells (7). We are confident that the mouse embryo can serve as in vivo model to investigate regulatory networks involved in development and disease and obtain data leading to discovery of novel drug targets and pharmaceutical compounds. Our project is in continuation with our previous efforts to identify genes involved in tissue and organ development at a large scale (funded in DHGP-II). We have identified a large number of new genes expressed in a restricted manner in the mid-gestation mouse embryo, which, in combination with published genes of that sort, provide valuable gene resources for a systematic analysis of regulatory networks involved in differentiation processes and disease (8; and unpublished data). Our gene expression database (MAMEP) will serve as resource in combination with public data and in silico promoter studies to identify genes involved in or downstream of the Wnt/ -cat, FGF and TGF 1/BMP signal cascades. In this project we aim at establishing new strategies for functional analysis of genes in vivo based on RNA interference in combination with tetraploid embryo/ES cell chimera technology allowing “large scale” functional analysis of genes in the mouse. This strategy will be instrumental in dissecting regulatory networks controlling embryonic processes such as EMT.
Project Status
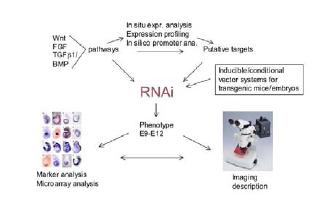
Fig 1: Schematic drawing representing principal steps of the work plan.
We plan to knock-down a large number of genes related to Wnt/ -cat, FGF and TGF 1/BMP signal cascades using vector based RNAi technology (shRNA vector systems) (9). Knock-down constructs are integrated either randomly or by recombinase mediated cassette exchange into the genome of embryonic stem cells. Efficacy of the shRNA constructs in RNA degradation can be tested in differentiating ES cells or in teratocarcinoma cells. ES cells harbouring constructs effecting RNA degradation of the target gene transcripts are used for the generation of embryo chimera via aggregation with recipient tetraploid embryos. Tetraploid embryos are generated by electro-fusion of the cells of a two-cell embryo. Such embryos have the unique property that they do not contribute to the formation of the embryo proper, but form only extra-embryonic membranes and tissues. Thus, chimera derived from tetraploid recipient embryos and ES cells form embryo propers which are exclusively derived from the ES cells and therefore have the construct, which was integrated into the ES cells, basically in all cells of the body. Since shRNA constructs act in a dominant manner, leading to degradation of the RNA derived from both gene copies, knock-down phenotypes can be obtained directly in tetraploid embryo/ES cell chimera. This precludes time consuming breeding steps such as required for the analysis of knock-out alleles which in general are recessive mutations. The disadvantage of this method is the fact that the dominant knock-down of a gene may lead to early embryo lethality which precludes generation of mutant lines. To overcome this problem we work on establishing inducible shRNA constructs. Such constructs would allow controlling the time, and eventually even time and tissue, when and where the gene of interest is knocked-down. This would overcome early lethality problems and also allow establishing conditional knock-down lines facilitating the in depth analysis of mutant phenotypes.
Phenotypes are first evaluated by morphological criteria, followed by expression profiling on gene chips. Finally, whole mount in situ analyses of sets of marker genes and of genes differentially regulated in the mutant as compared to the wild type allele are performed to verify expression profiling data. Knock-down of key factors in the Wnt/ -cat, FGF and TGF 1/BMP signal pathways are initially used to interrupt signalling and generate phenocopies of mutations in these pathways. At the moment we are in the process of selecting targets for knock-down, shRNA vector construction and establishing of ES cell lines carrying effective shRNA constructs.
Expression profiling of the resulting mutant embryos and marker gene analyses is used to identify candidate genes acting downstream of these pathways. Public data, gene expression data obtained by us during DHGP (MAMEP database) and in silico promoter analyses (SMP DNA) are utilized to predict additional factors involved in these pathways. The combined data serve to select appropriate genes for further knock-down analyses. Regulatory networks will be derived from the molecular characterization of knock down phenotypes based primarily on expression data of marker genes using whole mount in situ analyses, in combination with in silico promoter analysis data.
Outlook
Gene knock-down in embryos and mice will be developed to a high-throughput technology for functional analysis in the mouse. This will promote the dissection of regulatory networks controlling embryonic processes such as EMT and organ development as well as provide mouse models for human disease. The prospects of this technology as tool in biomedical research are more than promising, making development of this technology mandatory.
Lit.: 1. Baird A. Fibroblast growth factors: activities and significance of non-neurotrophin neurotrophic growth factors. Curr Opin Neurobiol. 1994 4:78-86. 2. Unsicker K and Krieglstein K. TGF-betas and their roles in the regulation of neuron survival. Adv Exp Med Biol. 2002 513:353-74. 3. Kozlovsky N et al. GSK-3 and the neurodevelopmental hypothesis of schizophrenia. Eur Neuropsychopharmacol. 2002 12:13-25.. 4. Caricasole A et al. The Wnt pathway, cellcycle activation and beta-amyloid: novel therapeutic strategies in Alzheimer's disease? Trends Pharmacol Sci. 2003 24:233-8. 5. Ridley A et al. Cell Migration: Integrating Signals from Front to Back. Science. 2003 302:1704-1709. 6. Grünert S et al. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Mol. Cell Biol. 2003 4:657-665. 7. Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. BioEssays. 2001 23:912- 923. 8. Neidhardt L et al. Large scale screen for genes controlling embryogenesis, using high-throughput gene expression analysis in mouse embryos. Mech Development. 2000 98:77-93. 9. Kunath T et al. Transgenic RNA interference in ES cell-derived embryos recapitulates a genetic null phenotype. Nature Biotech. 2003 21:559-561.


