
Introduction
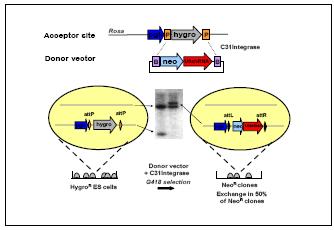
Fig 1: Site specific insertion of shRNA vectors into ES cells through RMCE.
The RNAi platform (SMP RNAi) aims to integrate RNAi as a tool to study gene function in human cells as well as in mouse as model organism for human biology. The efficacy of RNAi in mice has been studied in shRNA vector transgenic strains generated through pronucleus injection, viral embryo infection or from transfected ES cell clones (1-3). siRNAs, shRNA plasmids or lentiviral vectors were also directly delivered to somatic tissues by intravenous or intracerebral injection. These studies revealed that RNAi mediated gene silencing can be elicited at high efficiency in organs of embryonic and adult mice. Within the SMP RNAi in vivo section we develop RNAi into a standardised, scalable and time saving procedure for gene silencing in mice. This approach should enable us to produce phenotypic knock-down mutants at a larger scale and within less time as presently achieved with gene knock-out methodology. Furthermore, we aim at shortening the time window between the quest for gene function and its answer from in vivo studies. To this end, we establish and compare the production efficiency of transgenic RNAi mice based on ES cells or viral vectors with gene silencing induced through the viral infection of somatic tissues. In addition, we develop conditional methods for cell type-specific or inducible RNAi in mice. The best approach for the large scale production of RNAi mice will be used to generate mouse models for disease oriented research.
Project Status
For the ES cell based approach shRNA expression cassettes are inserted into the Rosa26 locus as defined genomic location through recombinase mediated cassette exchange (RMCE). We derive ES cell lines that contain recognition sites for the site specific recombinase C31-Integrase. One Rosa26 allele harbours the coding region of the hygromycin resistance gene flanked by a pair of attP recognition sites. These ES cells are transfected with an exogenous donor vector that contains a promoterless neo resistance linked to a shRNA expression vector together with a recombinase expression plasmid. Upon selection for neo resistance about half of the surviving clones contain a RMCE event (Fig. 1). Thus, only ~10 ES cell colonies are required to obtain several clones that contain a shRNA construct of chosen specificity (Fig. 2). Subsequently the modified ES cells are introduced into diploid blastocysts to generate germ line chimaeras or aggregated with tetraploid embryos to produce completely ES cell derived mice.
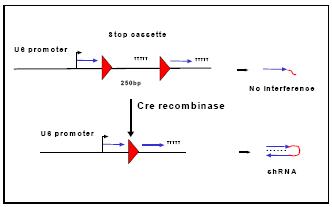
Fig 2: Activation of a conditional shRNA vector through Cre mediated recombination.
By the use of shRNA expression vectors that are activated through Cre recombinase conditional RNAi will allow inducible or cell type-specific gene silencing in mice. The temporal and spatial control of gene inactivation avoids embryonic lethality and permits to dissect gene function at high precision. For this purpose, a loxP flanked DNA segment is inserted into the promoter or into the shRNA loop region of the expression vector. This insertion includes a transcriptional stop signal that prevents the production of the full length hairpin RNA. Upon expression of Cre the inhibitory segment is excised from the vector and enables the production of the active shRNA (Fig. 2). Mice derived from such ES cells will be crossed to Cre transgenic strains exhibiting neuron specific recombinase expression.
In order to validate the described concept we initated a pilot experiment of 8 transgenic mouse strains harbouring either constitutive or conditional shRNA vectors (Table 1). The target genes are selected to reproduce a known knockout phenotype (Wnt, megane, CRHR1) or comprise signal transduction molecules for which the knockout phenotype in adults, due to embryonic lethality, is yet unknown (Braf, Mek1/2, GSK3a/ß). At present these shRNA strains are still under development and will be functionally analysed early in 2006.
Since we use ES cell lines harbouring a F1 hybrid genotype we have the additional option to produce completely ES cell derived (ES) mice by the aggregation of modified ES cells with tetraploid 4-cell embryos. We tested ES cell clones containing various shRNA expression vectors and found that most of them were able to generate ES mice at an efficiency of 5-12%.
Outlook
Upon a 18 months phase required to establish the various technologies we will apply the best suited method to set up a pipeline for the production of RNAi mouse models. A minimum of 25 knockdown strains will be generated to serve the study of CNS disorders and other diseases in the disease model project of the SMP RNAi.
Lit.: 1. Kunath T et al. Transgenic RNA interference in ES cell-derived embryos recapitulates a genetic null phenotype. Nat Biotechnol. 2003 21:559-561. 2. Hasuwa H et al. Small interfering RNA and gene silencing in transgenic mice and rats. FEBS Lett. 2002 532:227-230. 3. Rubinson D et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003 33:401-406.


